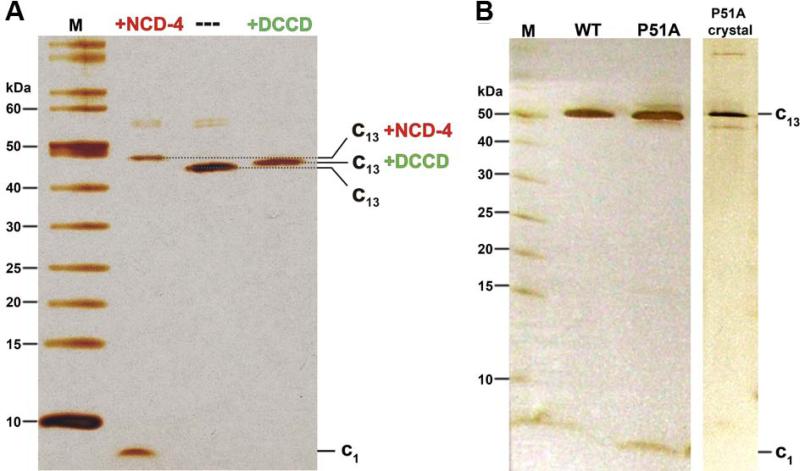
Figure 3. SDS-PAGE of purified B. pseudofirmus OF4 c13 ring samples.
(A) SDS-PAGE before and after reaction with the ATP synthase inhibitor N,N’-dicyclohexylcarbodiimide (DCCD) and its fluorescent derivative N-cyclohexyl-N'-(4-(dimethylamino)naphthyl)carbodiimide (NCD-4). A sample of ~0.4 μg purified B. pseudofirmus OF4 c13 ring was incubated with 0.4 mM NCD-4 or 0.5 mM DCCD for 24 h at pH 8.0 and then analyzed on 11% SDS-PAGE. The 13 covalently modified c-subunits (all reacted with E54) resulted in an increased molecular mass of the c13 ring, which was visualized by a slower migration on the silver-stained SDS-PAGE (lanes: +NCD-4 and +DCCD), as compared to an untreated c-ring sample (lane: ---). (B) SDS-PAGE purified B. pseudofirmus OF4 WT c13 ring, P51A c-ring and P51A c-ring crystals. All samples appear at the same level, suggesting that they all have a c13 stoichiometry, as confirmed later by the crystal structure. All gels were performed using the Schägger system (Schägger & von Jagow, 1987) and stained with silver. Molecular weight markers (M) in kDa are indicated.