Fig. 2.
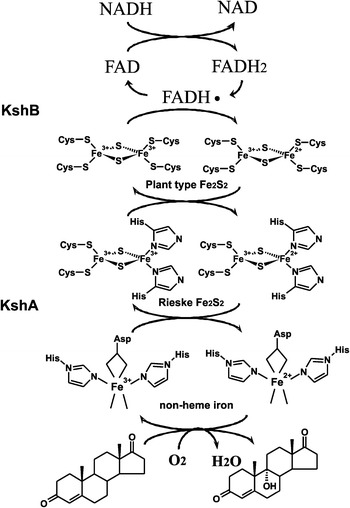
Electron transport chain reactions and substrate hydroxylation catalyzed by KSH. Arrows indicate the flow of electrons, starting from the electron donor NADH to the flavin co-factor of KshB, FAD, and via the plant type iron–sulphur cluster, coordinated by four cysteines, of KshB to the Rieske iron–sulphur cluster, coordinated by two cysteines and two histidines, of KshA ending up at the non-heme iron at the core of the catalytic domain. Non-heme iron is coordinated by an aspartate and two histidine residues leaving two binding sites open. Here O2 can be bound and one O-atom is used for the hydroxylation of the steroid substrate while the other O-atom is reduced to H2O (Petrusma 2011)
