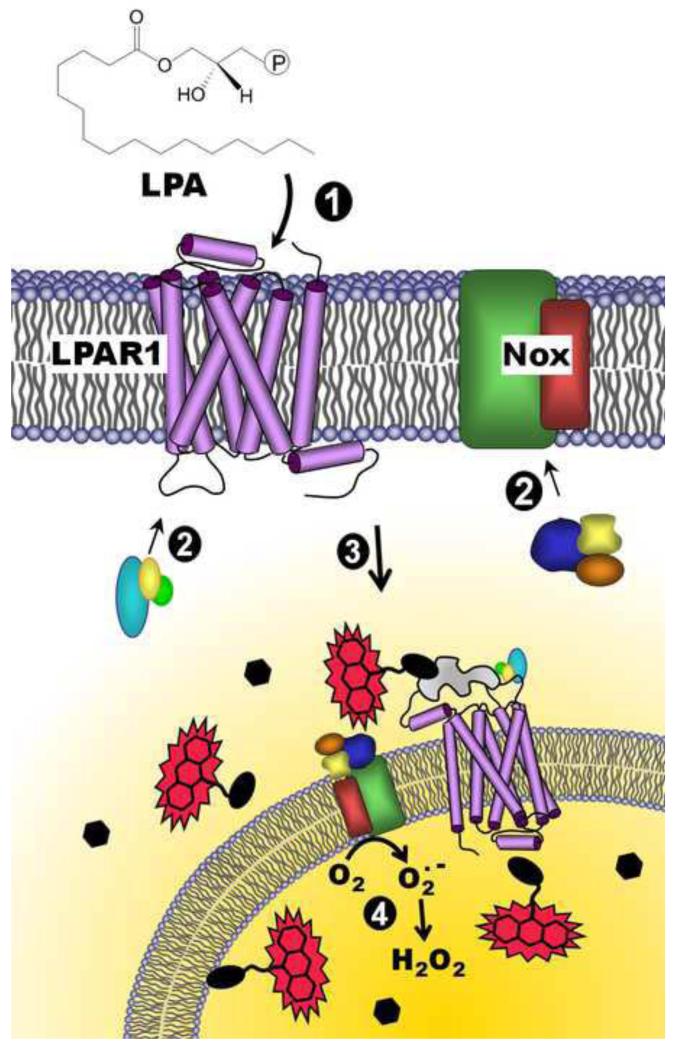
Figure 7.
Model for redox-dependent signaling through LPAR1 and depiction of the DCP-Rho1 labeling results demonstrating localized oxidation of proteins. Lysophosphatidic acid (LPA) binds to the extracellular portion of the LPA receptor (e.g. LPAR1) (1), resulting in the recruitment of G-proteins to the receptor (2, left) and co-stimulation of NADPH oxidase(s) (Nox) through association of cytosolic organizer and activator proteins (2, right). Together, activated LPAR1 and Nox complexes are internalized (3), and superoxide is produced by Nox inside the endosome (4). H2O2 generated by the spontaneous or enzyme-catalyzed dismutation of superoxide, which can diffuse out of the endosome, can then oxidize thiolate (R-S−)-containing proteins within or near these “redoxosomes”, generating the sulfenic acid modification (R-SOH). Upon addition of a labeling reagent, DCP-Rho1, which targets sulfenic acids, proteins containing this modification are covalently modified and associated with the rhodamine fluorophore, allowing detection following fixation, permeabilization and extensive washing to remove unbound DCP-Rho1. Black ellipses represent redox-sensitive proteins, and hexagons represent proteins which are insensitive to oxidation by H2O2. The irregular gray shape represents a scaffolding protein such as β-arrestin.