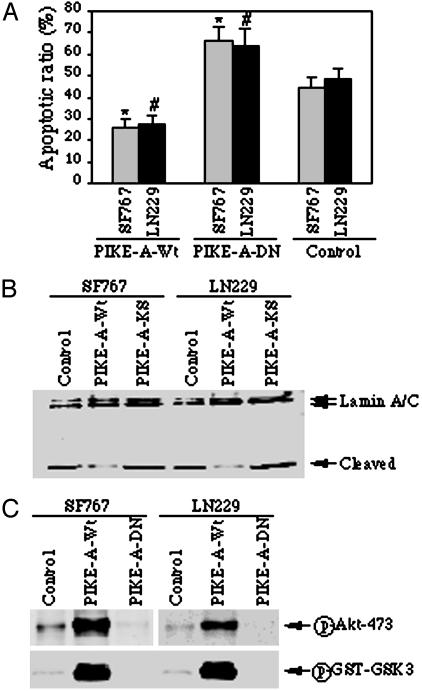
Fig. 4.
Overexpression of PIKE-A prevents apoptosis by stimulating Akt. (A) Apoptotic assay. Glioblastoma cells (SF767 and LN229) were infected with control adenovirus or adenovirus expressing wild-type PIKE-A or dominant-negative PIKE-A-DN, and treated with staurosporine for 16 h, and followed by chromatin condensation and fragmentation analysis with DAPI staining. Wild-type PIKE-A markedly prevents apoptosis compared to control cells (*, P < 0.005, Student's t test). Conversely, dominant-negative PIKE-A-DN increases the sensitivity of the infected cells to apoptosis (#, P < 0.01, Student's t test). (B) Western blotting analysis of Lamin A/C cleavage during apoptosis. Cell lysate (50 μg) from adenovirus-infected cells was separated by SDS/10% PAGE and analyzed by Western blotting with anti-lamin A/C antibody. Wild-type PIKE-A noticeably prevents lamin A/C cleavage compared to control cells. In contrast, dominant-negative PIKE-A-DN enhances lamin A/C cleavage. (C) PIKE-A stimulates Akt activity in glioblastoma cells. SF767 and LN229 cells were infected with adenovirus expressing PIKE-A-WT and PIKE-A-DN, respectively; after 24-h infection, the cell lysate was analyzed with phospho-serine-473 antibodies. Robust phosphorylation on S473 of Akt was observed in wild-type PIKE-A but not control adenovirus-infected cells. By contrast, the phosphorylation was completely inhibited by dominant-negative PIKE-A-DN (Upper). The in vitro Akt kinase assay was performed with the immunoprecipitated endogenous Akt, and GST–GSK3 was used as a substrate. GSK3 phosphorylation was analyzed by Western blotting with anti-phospho-GSK3-specific antibody (Lower).