Abstract
Objective:
To determine the incidence of immediate and delayed adverse drug reactions (ADRs), and to assess patient discomfort following administration of iodixanol during imaging examinations in routine clinical practice.
Methods:
A total of 20 185 patients across 95 clinical centres were enrolled in a prospective post-marketing surveillance registry with iodixanol. Patients were monitored for occurrence of ADRs immediately following iodixanol administration and for up to 7 days after administration.
Results:
The overall rate of ADRs was 1.52%, of which 0.58% was immediate and 0.97% was delayed onset. Two patients had non-fatal serious ADRs (0.01%). The ADRs were significantly more common in patients who underwent contrast-enhanced CT/coronary CT angiography vs others (p < 0.001), in those receiving pre-heated iodixanol vs non-heating (p < 0.001), in those aged 70 years or younger (p < 0.001), in those in whom a power injector was used for contrast delivery (p < 0.001) and in those with a history of an allergic reaction to contrast (p = 0.024). Multivariate analysis showed that female gender, intravenous route of contrast injection, body weight ≥80 kg, age less than 65 years, contrast flow rate ≥4 ml s−1 and prior reaction to iodinated contrast medium were all significant and independent contributors to ADRs. Pre-treatment contrast volume and history of cardiac disease, gout, hypertension, diabetes mellitus or asthma did not affect the rate of ADRs. Discomfort was generally mild, with 94.8% of patients reporting a composite score of 0–3.
Conclusion:
The safety of iodixanol in routine clinical practice was shown to be similar to the published safety profiles of other non-ionic iodinated contrast agents. Patient discomfort during administration was mild or absent in most patients.
Advances in knowledge:
The major strength of this study is that it included 20 185 patients enrolled in various types of imaging examinations. The safety profile of iodixanol was comparable to previously published work.
Iodinated contrast media (ICMs) are widely administered for diagnostic and interventional procedures. Despite their generally excellent safety record, adverse drug reactions (ADRs) still occur, ranging from minor disturbances to fatal complications, in very rare cases.1,2
Because of relatively small numbers of patients included in controlled clinical trials for initial drug approval, it is usually difficult to determine the true occurrence and frequency of ADRs associated with a drug. Post-marketing surveillance (PMS) studies, therefore, provide an opportunity to obtain further knowledge of safety and tolerability of an approved drug, including ICMs, in a “real-world” setting. The framework of a PMS programme enables prospective data to be collected from a very large non-selected patient population.3
The primary purpose of this study was to determine the ADR profile of iodixanol as used in routine clinical practice. Additionally, patient comfort profile following iodixanol administration was assessed.
METHODS AND MATERIALS
Study design
This was a prospective, non-interventional, non-randomized, multicentre, open-label, observational study carried out across 95 centres in China from June 2011 to October 2012. The study was registered at ChiCTR-ONC (11003061) and was sponsored by GE Healthcare, Shanghai, China.
Investigational review board approval was obtained where it was required. The study was conducted in full accordance with the principles of the Declaration of Helsinki and the good clinical practice (GCP) guidelines and adhered to the regulatory requirements and laws of China. The study population was clearly defined in the clinical study protocol along with other rigorous unambiguous inclusion/exclusion criteria. The principal investigator at each site was responsible for recruiting eligible patients to the trial and was to abide by the protocol throughout the course of the study. Data management and statistical analyses were conducted by an independent clinical research organization (Hangzhou Tigermed Consulting Co., Ltd, Hangzhou, China).
Observational plan
The safety surveillance started immediately following iodixanol administration and continued for 7 days after ICM administration. A standardized questionnaire, which permitted the systematic and integrated analysis of the data, was used to collect occurrence of ADRs. Inclusion and exclusion criteria of the protocol were consistent with the indications and contraindications specified in the local package insert. Written informed consent was obtained for every subject, either from the subject themselves or from a legally acceptable representative, before any procedure or assessment was done—after the aims, methods, anticipated benefits and potential hazards were explained. Iodixanol was administered in a routine manner according to the diagnostic indication and need or consistent with each institution's practising protocol. Pre-treatment (such as H1 antagonist or H2 antagonist and steroid) and the type of pre-treatment were based on the institutional standard. No specific criterion was established in the study.
Observational variables
The following data were collected from each patient and recorded in case report forms: patient demographics in conformance with local regulations, medical history and relevant underlying diseases (e.g. allergies, diabetes mellitus (DM), renal insufficiency, coronary heart disease, proteinuria, gout and arterial hypertension), pre-medication, type of examination/intervention, volume and dose of iodixanol administered, route of administration (intra-arterial (i.a.) or intravenous (i.v.)), status of vein/artery punctured, mode of injection (manually or using power injector), pre-heating iodixanol to body temperature before administration or not and overall tolerance to ICM.
Any immediate ADR (occurred within 1 h after ICM administration)4 or delayed ADR (occurred >1 h to 7 days after ICM administration)5 was recorded in the separate questionnaire regarding the type of signs/symptoms, onset time, lasing duration, severity, causal relationship and outcome. In addition, each ADR was evaluated for seriousness and was designated as a serious ADR if it met one of the following criteria: fatal or life-threatening, leading to significant or permanent damage/impairment or requiring hospitalization in an intensive care unit, hospital stay prolongation or leading to congenital anomalies. For those patients who experienced an ADR after iodixanol administration, the additional data were documented, including history of allergies, other relevant anamnestic features, previous ICM examinations, previous reactions to ICM, concomitant diseases (except the indication) and concomitant medications. No laboratory tests were required or collected.
Injection-associated patient discomfort was enquired about immediately after administration of iodixanol. Patients were asked to report and rate discomfort, such as pain at the injection site and sensations of coldness or heat in the injected vessel. Scores were reported verbally by the patient on a scale from 0 (no discomfort) to 10 (severe discomfort).
Statistical plan and analysis
The study sample size of approximately 20 000 was estimated based on previously published statistical calculations for PMS studies, which allows rare ADRs to be studied with an incidence of approximately 1.5/10 000.4,6
Data analysis was performed using SAS® software v. 9.2 (SAS Institute Inc., Chicago, IL). Patient data obtained from all sites were pooled into an integrated database. Data were analysed using descriptive statistical methods (e.g. frequency tables and descriptive statistical parameters). Pearson's χ2 test was used to analyse differences between different groups. Multivariants logistic regression analysis was performed to assess independent contributing factors to ADRs.
RESULTS
Patient demographics and baseline characteristics
A total of 20 185 unselected patients were included in the study during a 16-month period. Patient demographic data and baseline characteristics are shown in Table 1. The mean age of patients was 60.4 ± 12.8 years, with 42.8% of them between 51 and 65 years. 65% of the patients included in the study had one or more risk factors or the presence of underlying disease. Using criteria described by the Contrast Media Safety Committee of the European Society of Urogenital Radiology 7.0,7 65.4% of patients (13 203/20 185) reported 1 or more risk factors for contrast administration. The details on the risk factors are presented in Table 2. Pre-medication was administered to 2794 patients (13.8%), with the most common one being steroid (12.4%); H1 antagonist accounted for only 0.6%.
Table 1.
Patient demographic and baseline characteristics (all enrolled patients)
| Characteristics | n (%) |
|---|---|
| Total patient population | 20 185 |
| Male | 12 734 (63.1) |
| Female | 7451 (36.9) |
| Mean age (years) | 60.4 |
| Age range (years) | 5–100 |
| Age range (years) | |
| <18 | 9 (0.0) |
| 18–35 | 638 (3.2) |
| 36–50 | 3746 (18.6) |
| 51–65 | 8646 (42.8) |
| >65 | 7146 (35.4) |
| Mean body weight (kg) | 66.59 |
Table 2.
Patients with baseline risk factors (all enrolled patients)
| Characteristics | n (%) |
|---|---|
| Patients with risk factors | |
| Yes | 13 203 (65.4) |
| No | 6982 (34.6) |
| Impaired renal function | 435 (2.2) |
| Prior kidney surgery | 68 (0.3) |
| Gout | 130 (0.6) |
| Hypertension | 8333 (41.3) |
| Heart insufficiency | 825 (4.1) |
| Coronary heart disease | 5555 (27.5) |
| Diabetes requiring treatment | 2796 (13.9) |
| Asthma | 67 (0.3) |
| Previous moderate–severe reaction to ICM | 16 (0.1) |
| >70 years old | 4944 (24.5) |
ICM, iodinated contrast medium.
Type of examination and usage of iodixanol
The most common type of imaging examinations was contrast-enhanced CT (CECT) (25.6%), followed by coronary angiography (CAG) (20.4%), percutaneous coronary intervention (PCI) (19.5%), coronary CT angiography (CCTA) (17.8%) and interventional radiology (16.8%) (Table 3).
Table 3.
Examination type and iodixanol administration information (all enrolled patients)
| Parameter | Statistics | n (%) |
|---|---|---|
| Examination type | CECT | 5158 (25.6) |
| CCTA | 3585 (17.8) | |
| Diagnostic CAG | 4114 (20.4) | |
| CAG + PCI | 3940 (19.5) | |
| IR | 3391 (16.8) | |
| Iodixanol concentration | 270 mg I ml−1 | 379 (1.9) |
| 320 mg I ml−1 | 19 806 (98.1) | |
| Contrast pre-heated to body temperature | Yes | 8760 (43.4) |
| No | 11 425 (56.6) | |
| Status of artery/vein | Good | 18 093 (89.6) |
| Moderate | 1898 (9.4) | |
| Poor | 174 (0.9) | |
| Contrast volume (ml) | Mean | 95.9 |
| Min–max | 20–600 | |
| Contrast volume category (ml) | 0–50 | 2838 (14.1) |
| 51–80 | 6727 (33.3) | |
| 81–100 | 7321 (36.3) | |
| >100 | 3299 (16.3) | |
| Administration route | Artery | 11 049 (54.7) |
| Vein | 9112 (45.1) | |
| Othera | 24 (0.1) | |
| Injection mode | Manual | 7238 (35.9) |
| Automatic | 12 653 (62.7) | |
| Manual and automatic | 293 (1.5) | |
| Otherb | 1 (0.0) | |
| Flow rate (ml s−1) | Mean | 4.28 |
| Min–max | 1.0–25.0 | |
| Flow rate category (ml s−1) | ≤2 | 408 (2.0) |
| 2–3 | 4071 (20.2) | |
| 3–4 | 2908 (14.4) | |
| 4–5 | 3531 (17.5) | |
| >5 | 1766 (8.7) |
CAG, coronary angiography; CCTA, coronary CT angiography; CECT, contrast-enhanced CT; IR, interventional radiology; max, maximum; min, minimum; PCI, percutaneous coronary intervention.
Administration route: other included oral, oesophageal, biliary tract, subcutaneous, or percutaneous bilateral renal calyces administration.
Injection mode: other is oesophageal dosing.
Iodixanol 320 mg I ml−1 was the most frequently used concentration (in 98.1% of examinations). The route of administration was i.v. in 9112 (45.1%) cases, i.a. in 11 049 (54.7%) of the cases and by other means in 24 (0.1%) of the cases. The contrast was most frequently delivered by a power injector (62.7% of procedures), followed by manual injection (35.9%) and manual plus power injection (1.5%). The mean (±standard deviation) volume of iodixanol was 95.9 (±46.2) ml with a median flow rate of 4.28 ml s−1. In 43.4% patients, iodixanol was warmed to 37 °C prior to use. The status of punctured arteries/veins during the examination was“good” in 18 093 (89.6%) patients, “moderate” in 1898 (9.4%) patients and “poor” in 174 (0.9%) patients (Table 3).
Adverse drug reactions
Overall summary of adverse drug reactions
The overall incidence of ADRs in the study was 1.52% (307/20 185). Of which, immediate ADRs were 0.58% (117/20 185) and delayed ADRs were 0.97% (195/20 185). Five patients developed both types of reactions, and two patients had serious ADRs (0.01%) (Table 4). The two patients with serious ADRs had acute anaphylactic shock that resolved after treatment. There were no permanent injuries or deaths reported in any of the patients. The most common immediate ADRs were gastrointestinal disorders (n = 45, 0.22%), followed by skin and subcutaneous tissue disorders (n = 39, 0.19%) and nervous system disorders (n = 12, 0.06%). For the delayed ADRs, skin subcutaneous tissue disorders, including rash, pruritus, skin and mucosa erythema, occurred in 138/20 185 patients (0.68%), with rash being the highest (0.39%, 78/20 185 cases), followed by general disorders and administration site conditions, including pyrexia and face oedema in 21/20 185 cases (0.10%), then immune system disorders, including hypersensitivity, in 18/20 185 patients (0.09%).
Table 4.
Summary of adverse drug reactions (ADRs) by system organ class/preferred terms and onset time (incidence ≥0.05%) (all enrolled patients)
| Parameter | Immediate onset of ADRs, n (%) | Delayed onset of ADRs, n (%) | Both onsets of ADRs, n | Total no. of events |
|---|---|---|---|---|
| Patients with at least one ADR | 117 (0.58) | 195 (0.97) | 5 | 371 |
| Skin and subcutaneous tissue disorders | 39 (0.19) | 138 (0.68) | 3 | 195 |
| Rash | 15 (0.07) | 78 (0.39) | 2 | 93 |
| Hives | 12 (0.06) | 18 (0.09) | 1 | 30 |
| Pruritus | 5 (0.02) | 11 (0.05) | 0 | 16 |
| Mucocutaneous rash | 1 (0.00) | 11 (0.05) | 0 | 12 |
| Swelling face | 0 | 10 (0.05) | 0 | 10 |
| Immune system disorders | 5 (0.02) | 18 (0.09) | 1 | 23 |
| Hypersensitivity | 3 (0.01) | 17 (0.08) | 0 | 20 |
| Gastrointestinal disorders | 45 (0.22) | 9 (0.04) | 3 | 56 |
| Nausea | 27 (0.13) | 4 (0.02) | 2 | 31 |
| Vomiting | 16 (0.08) | 2 (0.01) | 1 | 18 |
| General disorders and administration site conditions | 11 (0.05) | 21 (0.10) | 1 | 36 |
| Nervous system disorders | 12 (0.06) | 11 (0.05) | 2 | 27 |
| Dizziness | 7 (0.03) | 7 (0.03) | 0 | 14 |
| Respiratory, thoracic and mediastinal disorders | 5 (0.02) | 4 (0.02) | 0 | 11 |
Incidences of adverse drug reactions in subgroups
There were notable differences in ADR rates in the different types of examinations: it was significantly higher for CCTA/CECT than for other procedures (p < 0.001) (Table 5). Among patients aged older than 18 but less than 70 years, the incidence of ADRs was higher than in those aged over 70 years (1.76% vs 0.69%, p < 0.001) (Table 6; Figure 1). Further age group analysis showed that significantly more ADRs were reported in the 18- to 40-year group (2.42%) vs the 41- to 70-year group (1.7%) and aged over 70 years group (0.76%) (p = 0.0001). The incidence of ADRs in patients who received pre-heated iodixanol was higher than in those who received non-heated iodixanol (1.84% vs 1.28%, p = 0.001). For patients with a “good” status of punctured vessels, the ADR rates were significantly higher in patients with punctured veins than in those with punctured arteries (p < 0.001). The ADR rates were higher in patients with “poor” status of punctured veins than in those with “moderately” punctured veins (5.81% vs 1.70%, p = 0.023). Significant differences in ADR rates were found with different contrast delivery methods: use of a power injector was associated with 1.98% of ADRs; manual and power injection, 1.02%; and manual injector, 0.73% (p < 0.001). The incidence of ADRs in patients who received <50 ml iodixanol was 1.34%; 50–100 ml was 1.62%; and >100 ml was 1.24%. The differences among the three dose groups were not statistically significant (p > 0.05) (Table 6).
Table 5.
Incidence of adverse drug reactions (ADRs) by examination type
| Examination type | Parameter | Patients with ADRs, n (%) | 95% CI (%) |
|---|---|---|---|
| CECT, N = 5158 | ADR | 111 (2.15) | 1.756, 2.548 |
| Serious ADR | 0 | ||
| Immediate onset | 42 (0.81) | 0.569, 1.060 | |
| Delayed onset | 70 (1.36) | 1.041, 1.673 | |
| CCTA, N = 3585 | ADR | 114 (3.18) | 2.606, 3.754 |
| Serious ADR | 0 | ||
| Immediate onset | 45 (1.26) | 0.891, 1.620 | |
| Delayed onset | 72 (2.01) | 1.549, 2.468 | |
| Diagnostic CAG, N = 4114 | ADR | 27 (0.66) | 0.410, 0.903 |
| Serious ADR | 0 | ||
| Immediate onset | 11 (0.27) | 0.110, 0.425 | |
| Delayed onset | 17 (0.41) | 0.217, 0.609 | |
| CAG + PCI, N = 3940 | ADR | 35 (0.89) | 0.595, 1.181 |
| Serious ADR | 2 (0.05) | 0.000, 0.121 | |
| Immediate onset | 14 (0.36) | 0.170, 0.541 | |
| Delayed onset | 21 (0.53) | 0.306, 0.760 | |
| IR, N = 3391 | ADR | 21 (0.62) | 0.355, 0.883 |
| Serious ADR | 0 | ||
| Immediate onset | 6 (0.18) | 0.035, 0.318 | |
| Delayed onset | 15 (0.44) | 0.219, 0.666 |
CAG, coronary angiography; CCTA, coronary CT angiography; CECT, contrast-enhanced CT; CI, confidence interval; IR, interventional radiology; PCI, percutaneous coronary intervention.
Immediate onset—occurring within 1 h of the administration of iodixanol.
Delayed onset—occurring after 1 h up to 7 days after administration of iodixanol.
Table 6.
Comparison of incidences of adverse drug reactions (ADRs) in different groups (all enrolled patients)
| Variables | Incidences of ADRs, n (%) | p-value |
|---|---|---|
| Age (years) | ||
| ≤70 | 247 (1.89) | |
| >70 | 60 (0.84) | <0.001 |
| Dose (ml) | ||
| <50 vs 50–100 | 38 (1.34) vs 228 (1.62) | 0.284 |
| <50 vs >100 | 38 (1.34) vs 41 (1.24) | 0.735 |
| 50–100 vs >100 | 228 (1.62) vs 41 (1.24) | 0.118 |
| Status of arterial puncturing | ||
| Good vs moderate | 81 (0.79) vs 2 (0.28) | 0.177 |
| Good vs poor | 81 (0.79) vs 0 (0.00) | 1.000 |
| Moderate vs poor | 2 (0.28) vs 0 (0.00) | 1.000 |
| Status of venous puncturing | ||
| Good vs moderate | 199 (2.54) vs 20 (1.70) | 0.084 |
| Good vs poor | 199 (2.54) vs 5 (5.81) | 0.070 |
| Moderate vs poor | 20 (1.70) vs 5 (5.81) | 0.023 |
| Pre-heating | ||
| Yes | 161 (1.84) | |
| No | 146 (1.28) | 0.001 |
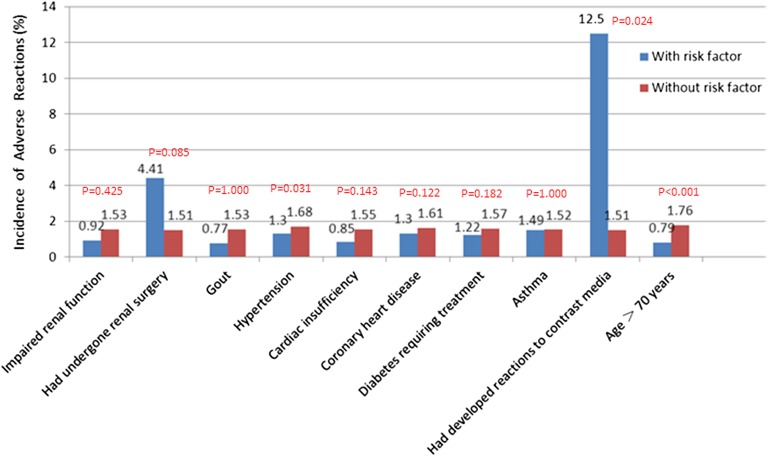
Figure 1.
Incidence of adverse drug reactions by risk factors.
Adverse drug reactions and risk factor correlations
Comparison of the ADR rates between patients with and without risk factors revealed that patients with a history of previous ICM reaction were associated with increased rates of ADR (p = 0.024). Patients with hypertension had a significantly lower rate of ADRs than those without hypertension (1.30% vs 1.68%, p = 0.031). There was no difference between the ADR rates of patients pre-treated with H1 and H2 antihistamines or corticosteroids and those without pre-treatment (1.72% with pre-treatment vs 1.49% without, p = 0.3595).
In the multivariate analysis for the ADRs to identify independent contributing factors, the following variables were included in the model: previous reaction to ICM (yes vs no), age category (<65 vs ≥65 years old), weight category (<80 vs ≥80 kg), gender (male vs female), contrast flow rate (<4 vs ≥4 ml s−1), administration route (artery vs vein or other route), total contrast volume (<100 vs ≥100 ml), contrast concentration (320 vs 270 mg I ml−1), pre-heating (yes vs no), kidney disease (yes vs no), cardiac disease (yes vs no), gout (yes vs no), DM (yes vs no) and asthma (yes vs no). The result of this analysis is presented in Table 7. Female gender, i.v. route of administration, age less than 65 years, body weight ≥80 kg, contrast flow rate ≥4 ml s−1 and prior reaction to ICM were identified as significant contributors to ADRs. None of the other variables was a significant factor.
Table 7.
Multivariable logistic regression analyses determining the effect of different factors on adverse drug reactions
| Variable | Estimate (SE) | OR | 95% CI | p-value |
|---|---|---|---|---|
| Iodixanol (mg I ml−1), 320 vs 270 | 13.4255 (307.2000) | >999.990 | <0.001, >999.990 | 0.9651 |
| Artery vs vein + othersa route | −1.6393 (0.2447) | 0.194 | 0.120, 0.314 | <0.0001 |
| Pre-heating, yes vs no | 0.2727 (0.1364) | 1.314 | 1.005, 1.716 | 0.0455 |
| Contrast volume (ml), ≥100 vs <100 | 0.0733 (0.1642) | 1.076 | 0.780, 1.485 | 0.6551 |
| Flow rate (ml s−1), ≥4 vs <4 | 0.6126 (0.1451) | 1.845 | 1.389, 2.452 | <0.0001 |
| Age (years), ≥65 vs <65 | −0.7694 (0.1691) | 0.463 | 0.333, 0.645 | <0.0001 |
| Gender, male vs female | −0.4780 (0.1391) | 0.620 | 0.472, 0.814 | 0.0006 |
| Weight (kg), ≥80 vs <80 | 0.5172 (0.1764) | 1.677 | 1.187, 2.370 | 0.0034 |
| Kidney disease, yes vs no | −12.2128 (2234.5000) | <0.001 | <0.001, >999.990 | 0.9956 |
| Cardiac disease, yes vs no | −0.4587 (0.7218) | 0.632 | 0.154, 2.601 | 0.5251 |
| Gout, yes vs no | 0.1156 (1.0213) | 1.123 | 0.152, 8.309 | 0.9098 |
| Hypertension, yes vs no | −0.0507 (0.1497) | 0.951 | 0.709, 1.275 | 0.7350 |
| Diabetes mellitus, yes vs no | −0.0379 (0.2353) | 0.963 | 0.607, 1.527 | 0.8721 |
| Asthma, yes vs no | 0.3316 (1.0212) | 1.393 | 0.188, 10.310 | 0.7454 |
| Prior reaction to ICM, yes vs no | 2.3264 (0.8078) | 10.241 | 2.102, 49.886 | 0.0040 |
CI, confidence interval; ICM, iodinated contrast media; OR, odds ratio; SE, standard error.
Administration route: other included oral, oesophageal, biliary tract, subcutaneous, or percutaneous bilateral renal calyces administration.
Patient discomfort after iodixanol contrast administration
Pain
A total of 94.3% (19 044/20 185) patients in this study had no pain after the injection of iodixanol, whereas 5.7% of the patients experienced various degrees of pain: 5.3% of these (1065/20 185 patients) had mild pain (score, 1–3), 0.4% (74/20 185) had moderate pain (score, 4–7) and only 2 patients had severe pain (score, 8–10) (Table 8).
Table 8.
Summary of patient discomfort
| Discomfort, category/score | Pain, n (%) | Heat, n (%) | Cold, n (%) | Composite score | Number of patients |
|---|---|---|---|---|---|
| None/0 | 19 044 (94.3) | 15 288 (75.7) | 19 806 (98.1) | 0 | 14 791 (73.3) |
| Mild/1–3 | 1065 (5.3) | 4134 (20.5) | 347 (1.7) | 1–3 | 4338 (21.5) |
| Moderate/4–7 | 74 (0.4) | 736 (3.6) | 29 (0.1) | 4–15 | 1054 (5.2) |
| Severe/8–10 | 2 (0.0) | 27 (0.1) | 3 (0.0) | >15 | 2 (0.0) |
Heat
In this study, 75.7% of patients (15 288/20 185) experienced no heat sensation after injection of iodixanol, whereas 24.3% of them had various degrees of heat sensation: 20.5% (4134 patients) had mild (score, 1–3), 3.6% (736 patients) had moderate (score, 4–7) and 0.1% (27 patients) had severe (score 8–10) heat sensation (Table 8).
Coldness
Most patients (19 806/20 185, 98.1%) did not report feeling cold at all. Only 1.9% of them had various degrees of feeling cold: 1.7% (347 patients) had mild (score, 1–3), 0.1% (29 patients) had moderate (score, 4–7) and 3 patients had severe (score, 8–10) cold sensation (Table 8).
Composite score
The individual categorical scores (0–10) for pain, coldness and heat were combined to form a composite score (up to 30). Score 0, i.e. no discomfort, was seen in 14 791/20 185 patients (73.3%), score 1–3 in 4338/20 185 patients (21.5%), score 4–15 in 1054/20 185 patients (5.2%) and a composite score >15 was seen in 2 patients in this study (Table 8). In patients with a “poor” status of punctured vessels, 71.8% (125/174 patients) reported some discomfort (composite score ≥1) compared with 45.9% (871/1898 patients) with a “moderate” and 24.3% (4393/28 093 patients) with a “good” status of punctured vessels. Patients who received pre-heated iodixanol were also more likely to report a composite score of 1–30 than those who received non-heated iodixanol (2972/8760 patients or 33.9% vs 2422/11 425 patients or 21.2%, respectively). In the assessment of composite score in the different types of examinations, a composite score of 1–30 was seen in 50.0% of CCTA examinations, followed by CECT (30.2%). A composite score of 1–10 was more frequently seen in the use of a power injector (4222/12 653 patients, 33.37%) than in the use of manual injection (1031/7238 patients, 14.24%).
DISCUSSION
We investigated the incidence of ADRs following the use of iso-osmolar non-ionic dimeric X-ray contrast agent, iodixanol, in 20 185 non-selected patients who underwent routine clinical imaging examinations at 95 centres in China. With this large database for iodixanol, we are able to understand its risk–benefit profile relative to other ICMs in use today.
The incidence of ADRs following non-ionic ICM administration has been reported ranging from 0.6% to 2.3% in most published studies,8,9 with one notable exception of 5.0% by Munechika et al5 after using iohexol for urography and CT. In our study, the overall ADR rate of 1.52% (immediate ADRs, 0.58%, and delayed ADRs, 0.97%, respectively) is in line with those reported for other non-ionic ICMs using a comparable methodology (Table 9).4–13 The occurrence of serious ADRs was extremely rare (0.01%) and comparable to the findings of a meta-analysis (0.031%),14 which compared non-ionic ICMs with ionic high-osmolar ICMs. There were statistically significant differences in the rates of ADRs for the route of administration and status of punctured veins. Despite lower average doses of iodixanol being administered through the i.v. route compared with the i.a. route, higher ADRs were reported in the i.v. administration group and in patients with a “poor” status of punctured veins, which supports some previous findings.4,15 In addition, the method of administration also appears to be related to occurrences of ADRs: more patients reported ADRs when iodixanol was administered via a power injector than via manual injections. This finding is also supported by other literature.4,16 We observed that patients undergoing CCTA or CECT had a significantly higher rate of ADRs than those undergoing CAG and/or PCI (p < 0.001, respectively). This finding may have arisen, in part, because patients undergoing CCTA or CECT received i.v. contrast medium injections. Patients in the age group 18–70 years experienced a higher rate of ADRs than those in the over 70 years age group. Further analysis revealed that more ADRs occurred in the 18–41 years age group, which is similar to previous reports.4,8 The higher rate of ADRs in the younger patient group might be attributable to a higher immunocompetence among younger adults, suggesting that an immune-mediated process is involved.8 This theory is also supported by the lack of any significant association of the ADRs with the concentration and dose of iodixanol.
Table 9.
Summary of adverse drug reactions (ADRs) following iodinated contrast media administrations in other clinical trials
| Reference | Procedure | Contrast medium | n | ADR rates (%) |
|
|---|---|---|---|---|---|
| Overall | Serious | ||||
| Kopp et al4 | X-ray examinations | Iopromide | 74 717 | 1.50a | 0.02 |
| Munechika et al5 | Intravenous urography or CECT | Iohexol | 7505 | 5.00b | / |
| Häussler6 | CECT | Iodixanol | 9515 | 0.74 | 0.05 |
| Petersein et al8 | Diagnostic procedures | Iobitridol | 61 754 | 2.30c | 1 died |
| Mortelé et al10 | CECT | Iopromide | 29 508 | 0.70 | / |
| Vogl et al11 | X-ray examinations | Iobitridol | 52 057 | 0.96 | 0.044 |
| Wendt-Nordahl et al12 | Intravenous urography | Iobitridol | 49 975 | 0.90d | 1 anaphylactic shock |
| Maurer et al9 | X-ray examinations | Iobitridol | 160 000 | 0.60 | / |
| Palkowitsch et al13 | X-ray examinations | Iopromide | 44 835 | 2.80 | 0.02 |
CECT, contrast-enhanced CT.
Acute reactions.
Delayed adverse reactions.
1.1% had “feeling of warmth”.
Acute adverse events (non-serious and transient).
Importantly, this large study enables us to identify contributing factors to ADRs. We found that ADRs were significantly more common in patients with a history of allergic reaction to ICMs (12.5% vs 1.51%; p = 0.024), which confirms that patients with allergies and/or prior hypersensitivity reactions to ICMs are indeed at an increased risk for developing ADRs. This is in agreement with Häussler,6 who found that patients with allergic diathesis appeared to be at an increased risk of immediate and delayed ADRs, and with Kopp et al,4 who reported significantly more immediate ADRs in this type of patient (7.4% vs 1.2%; p < 0.001). Patients with hypertension were found to have a lower rate of ADRs than those without, in the group comparison. However, this significant difference disappeared in the multivariant logistical regression analyses (p = 0.7386), presumably because it was just a confounding but not true contributing factor to ADRs.
It has been debatable whether pre-treatment can reduce ADRs among high-risk patients. Vogl et al11 did not find an effect of pre-treatment on the occurrence of adverse reactions in patients with a history of asthma and/or allergies, whereas Kopp et al4 reported a slight decrease in the overall adverse event rate, but the difference was not significant (1.6% without vs 1.4% with pre-treatment). This may be attributable to the fact that the underlying pathophysiology of allergic reactions is not yet fully understood; therefore, many allergic ICM reactions are unpredictable,17 and, therefore, unpreventable. It is fortunate that the ADR rate is very low following non-ionic ICM administration, even in high-risk patients, which is certainly confirmed by our study results.
The effect of pre-heating ICMs on ADR risk has been somewhat controversial. Vergara and Seguel18 reported a reduction in adverse events after the warming of i.v. ICM, whereas in another retrospective study,19 adverse event rates were not affected by warming iopamidol to body temperature prior to i.v. administration for CT. In our study, warming iodixanol to 37 °C prior to administration resulted in an increase in the number of ADRs. It is not clear to us what might be responsible for this phenomenon.
As with findings from previous studies,4 female patients in this study reported a significantly higher rate of ADRs than male patients [odds ratio (OR) = 0.621, 95% confidence interval (CI): 0.473, 0.815]. We also found that body weight (>80 kg) was an independent predictor of ADRs (OR = 1.657, 95% CI: 1.173, 2.341) along with a contrast flow rate ≥4 ml s−1 (OR = 1.846, 95% CI: 1.389, 2.452). These results are both clinically plausible and logical.
Discomfort characterized by pain, warmth and cold in connection with injection are common adverse effects associated with the use of intravascular ICMs. In some controlled clinical trials, more than one-third of patients reported injection-related discomfort, particularly local pain and an intense unpleasant sensation of warmth following ICM administration.20 The degree of discomfort and tolerability, generally considered to be directly proportional to the osmolality of the ICM, can influence the quality of the examination. Pain and discomfort may cause patients to move, thus resulting in motion artefacts and suboptimal images.21 Reduction in pain and discomfort is an important goal for improving the overall tolerability of any procedure. In line with earlier PMS study findings,6 a high proportion of patients tolerated iodixanol injection well in this study, with 73.3% of the patients feeling no discomfort at all (a composite score of 0 for pain, heat and coldness) and 21.5% reporting very mild discomfort (a composite score of 1–3 out of a possible total of 30). This result is consistent with the findings of many previous studies.6,9
The major strength of this study is that a large number of patients undergoing routine imaging examinations were included in various types of examinations across a large number of centres. This minimizes bias caused by single-centre and/or single-indication effects, and thus increases applicability of the study results to daily clinical practice. As a PMS study, it was also performed strictly according to GCP/International Conference on Harmonisation standards protecting participants' rights by obtaining informed consent either from patients directly or from their legal surrogates where the consent could not be obtained from the patient because of the use of sedative or anaesthetic drugs.
On the other hand, this study has some usual limitations inherent to the observational study, such as no randomization/blinding, no reference standard and no uniform standard to assess baseline risk factors and adverse events in the participating study centres.
In conclusion, iodixanol administration in 20 185 patients for various imaging examinations during routine clinical practice demonstrated a reassuring safety profile that is consistent with clinical trial data and previous clinical experience with ICM applications. The present study also confirmed that iodixanol is a well-tolerated contrast medium with an excellent comfort profile.
FUNDING
The study was sponsored by GE Healthcare, Shanghai, China.
ACKNOWLEDGMENTS
Editorial support was provided by Rubin Sheng, MD, MPH. The authors would like to express our enormous appreciation and gratitude to all participating sites.
REFERENCES
- 1.Bettmann MA. Frequently asked questions: iodinated contrast agents. Radiographics 2004; 24(Suppl. 1): S3–10. doi: 10.1148/rg.24si045519 [DOI] [PubMed] [Google Scholar]
- 2.Namasivayam S, Kalra MK, Torres WE, Small WC. Adverse reactions to intravenous iodinated contrast media: a primer for radiologists. Emerg Radiol 2006; 12: 210–15. doi: 10.1007/s10140-006-0488-6 [DOI] [PubMed] [Google Scholar]
- 3.Michel MC. Post-marketing studies can make important contributions to medical knowledge. BMJ 2012; 345: e4740. [DOI] [PubMed] [Google Scholar]
- 4.Kopp AF, Mortele KJ, Cho YD, Palkowitsch P, Bettmann MA, Claussen CD. Prevalence of acute reactions to iopromide: postmarketing surveillance study of 74,717 patients. Acta Radiol 2008; 49: 902–11. doi: 10.1080/02841850802282811 [DOI] [PubMed] [Google Scholar]
- 5.Munechika H, Hiramatsu Y, Kudo S, Sugimura K, Hamada C, Yamaguchi K, et al. A prospective survey of delayed adverse reactions to iohexol in urography and computed tomography. Eur Radiol 2003; 13: 185–94. doi: 10.1007/s00330-002-1339-9 [DOI] [PubMed] [Google Scholar]
- 6.Häussler MD. Safety and patient comfort with iodixanol: a postmarketing surveillance study in 9515 patients undergoing diagnostic CT examinations. Acta Radiol 2010; 51: 924–33. doi: 10.3109/02841851.2010.504739 [DOI] [PubMed] [Google Scholar]
- 7.Thomsen HS. European Society of Urogenital Radiology (ESUR) guidelines on the safe use of iodinated contrast media. Eur J Radiol 2006; 60: 307–13. doi: 10.1016/j.ejrad.2006.06.020 [DOI] [PubMed] [Google Scholar]
- 8.Petersein J, Peters CR, Wolf M, Hamm B. Results of the safety and efficacy of iobitridol in more than 61,000 patients. Eur Radiol 2003; 13: 2006–11. doi: 10.1007/s00330-002-1583-z [DOI] [PubMed] [Google Scholar]
- 9.Maurer M, Heine O, Wolf M, Freyhardt P, Schnapauff D, Hamm B. Safety and tolerability of iobitridol in general and in patients with risk factors: results in more than 160,000 patients. Eur J Radiol 2011; 80: 357–62. doi: 10.1016/j.ejrad.2010.03.018 [DOI] [PubMed] [Google Scholar]
- 10.Mortelé KJ, Oliva MR, Ondategui S, Ros PR, Silverman SG. Universal use of nonionic iodinated contrast medium for CT: evaluation of safety in a large urban teaching hospital. AJR Am J Roentgenol 2005; 184: 31–4. [DOI] [PubMed] [Google Scholar]
- 11.Vogl TJ, Honold E, Wolf M, Mohajeri H, Hammerstingl R. Safety of iobitridol in the general population and at-risk patients. Eur Radiol 2006; 16: 1288–97. doi: 10.1007/s00330-005-0061-9 [DOI] [PubMed] [Google Scholar]
- 12.Wendt-Nordahl G, Rotert H, Trojan L, Michel MS, Peters CR, Alken P, et al. Intravenous contrast media in uroradiology: evaluation of safety and tolerability in almost 50,000 patients. Med Princ Pract 2006; 15: 358–61. doi: 10.1159/000094269 [DOI] [PubMed] [Google Scholar]
- 13.Palkowitsch P, Lengsfeld P, Stauch K, Heinsohn C, Kwon ST, Zhang SX, et al. Safety and diagnostic image quality of iopromide: results of a large non-interventional observational study of European and Asian patients (IMAGE). Acta Radiol 2012; 53: 179–86. doi: 10.1258/ar.2011.110359 [DOI] [PubMed] [Google Scholar]
- 14.Caro JJ, Trindade E, McGregor M. The risks of death and of severe nonfatal reactions with high- vs low-osmolality contrast media: a meta-analysis. AJR Am J Roentgenol 1991; 156: 825–32. doi: 10.2214/ajr.156.4.1825900 [DOI] [PubMed] [Google Scholar]
- 15.Shehadi WH. Contrast media adverse reactions: occurrence, recurrence, and distribution patterns. Radiology 1982; 143: 11–17. doi: 10.1148/radiology.143.1.7063711 [DOI] [PubMed] [Google Scholar]
- 16.Jacobs JE, Birnbaum BA, Langlotz CP. Contrast media reactions and extravasation: relationship to intravenous injection rates. Radiology 1998; 209: 411–16. doi: 10.1148/radiology.209.2.9807567 [DOI] [PubMed] [Google Scholar]
- 17.Morcos SK. Review article: acute serious and fatal reactions to contrast media: our current understanding. Br J Radiol 2005; 78: 686–93. doi: 10.1259/bjr/26301414 [DOI] [PubMed] [Google Scholar]
- 18.Vergara M, Seguel S. Adverse reactions to contrast media in CT: effects of temperature and ionic property. Radiology 1996; 199: 363–6. doi: 10.1148/radiology.199.2.8668779 [DOI] [PubMed] [Google Scholar]
- 19.Davenport MS, Wang CL, Bashir MR, Neville AM, Paulson EK. Rate of contrast material extravasations and allergic-like reactions: effect of extrinsic warming of low-osmolality iodinated CT contrast material to 37 degrees C. Radiology 2012; 262: 475–84. doi: 10.1148/radiol.11111282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Swanson DP, Thrall JH, Shetty PC. Evaluation of intravascular low-osmolality contrast agents. Clin Pharm 1986; 5: 877–91. [PubMed] [Google Scholar]
- 21.Dawson P. The non-ionic isotonic contrast agents. Perspectives and controversies. Eur Radiol 1996; 6(Suppl. 2): S20–4. [DOI] [PubMed] [Google Scholar]