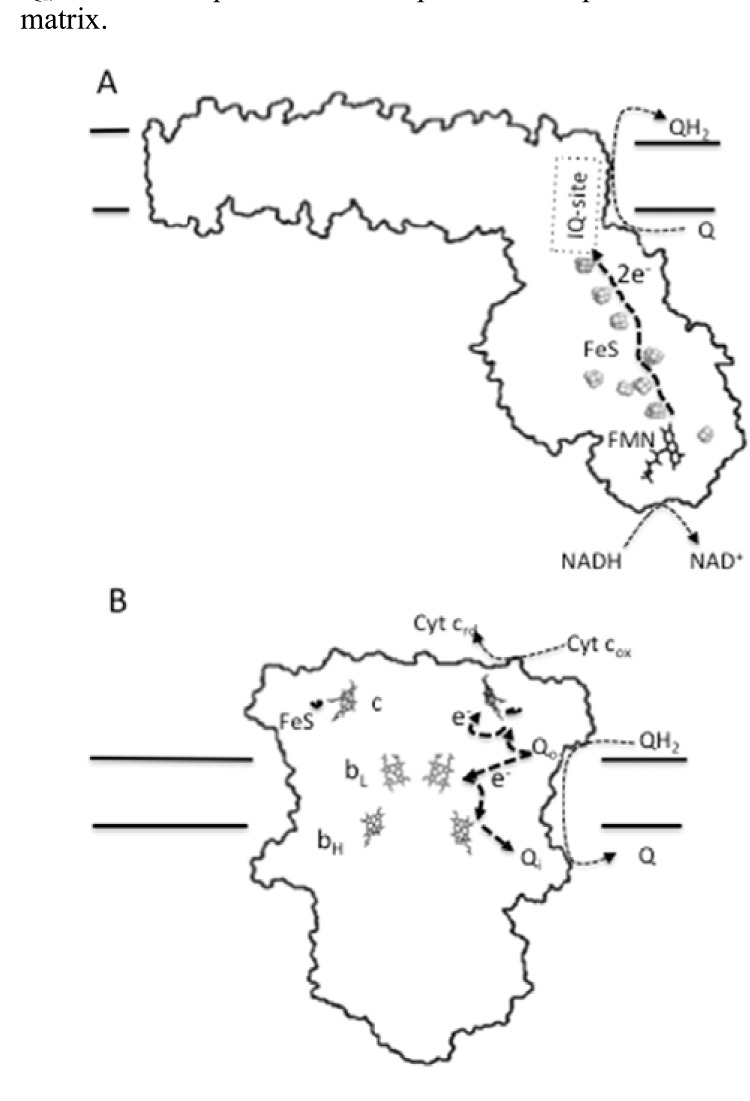
Fig. (3).
Prosthetic groups and redox centers of ROS-producing complexes I and III. A diagram of complex I indicating the pathway of electrons transfer from NADH to ubiquinone (A). The complex has a L-shaped structure, with a hydrophobic arm embedded into the inner mitochondrial membrane and a matrix-protruding hydrophilic arm, which contains three redox centers. The first center is formed by the flavin mononucleotide, FMN, prosthetic group (IF-site). The second center consists of nine FeS clusters. A third redox center of complex I is formed by the ubiquinone binding site (IQ-site). In (B) the arrangement of the redox centers of complex III is shown. This complex, which is a homodimer, passes electrons from ubiquinol to cytochrome c. Each monomer possesses three subunits with prosthetic groups that serve as redox centers. These are cytochrome b (which contains two hemes: bH and bL), cytochrome c1 (which contains one heme c group) and the Rieske iron-sulfur protein (which contains a 2Fe-2S cluster). In addition to these prosthetic groups, complex III has two separated ubiquinone/ubiquinol binding sites: an ubiquinol oxidizing site (Qo) and an ubiquinone reducing site (Qi), which, despite the lack of covalently bound prosthetic groups, are key factors of the redox process.