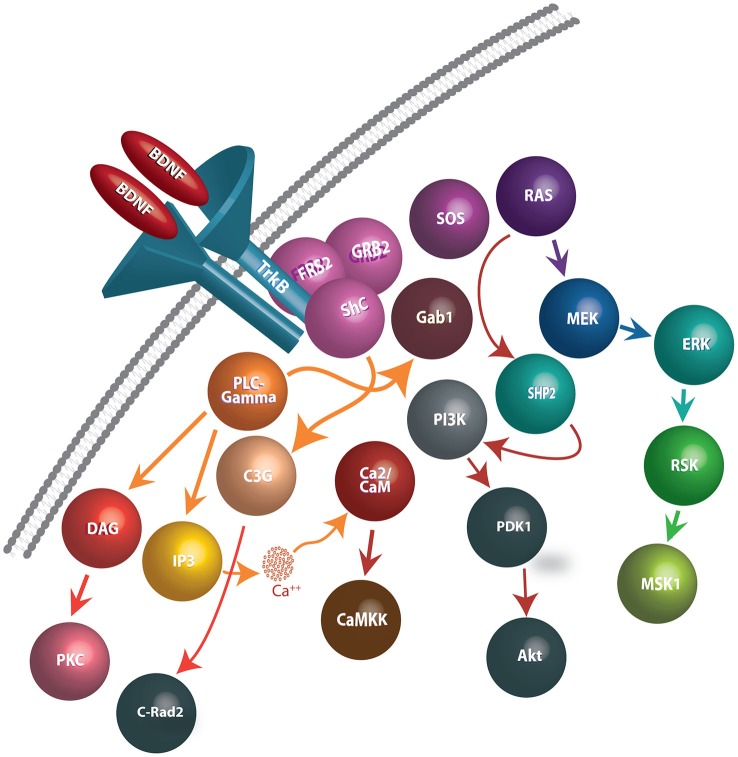
Figure 1.
Schematic representation of BDNF release, binding to TrkB receptors, and downstream events following internalization. BDNF dimers bind to TrkB receptors and cause autophosphorylation of tyrosine residues on the cytoplasmic domain of TrkB receptors, generating docking sites for several intracellular proteins. In turn, the activation of the TrkB receptors facilitate interactions with Shp2, Shc, and PLC-γmolecules and effectuates the signaling cascades such as PLC/PKC, PI3K/Akt, Ras/Erk, AMPK/ACC and NFB pathways. Signaling pathways involved in BDNF-TrkB interactions include: (1) PLC-γ1 signaling: Phosphorylated TrkB receptors bind to PLC-γ1 and lead to its’ activation. PLC-γ1 hydrolyses Phosphatidylinositol (4,5) to generate IP3 and DAG. While IP3 promotes release of Ca2+ from internal stores, DAG stimulates DAG-regulated protein kinase C isoforms. (2) Ras-MAP/erk signaling: Phosphorylation of Trk receptors provides a recruitment site for binding of the PTB domain of the adaptor protein, Shc. Shc recruits the adaptor protein, Grb2, and complexes with SOS, an exchange factor for Ras (and Rac). Activated Ras stimulates signaling through several downstream pathways, including those mediated by PI3-kinases, Raf, and p38MAP kinase. (3) PI-3 kinase signaling: Phosphatidylinositides are generated by PI3-kinase and activate phosphatidylinositide-dependent protein kinase (PDK-1). PDK-1 activates the protein kinase Akt (also known as PKB), which then phosphorylates several proteins important in promoting cell survival.