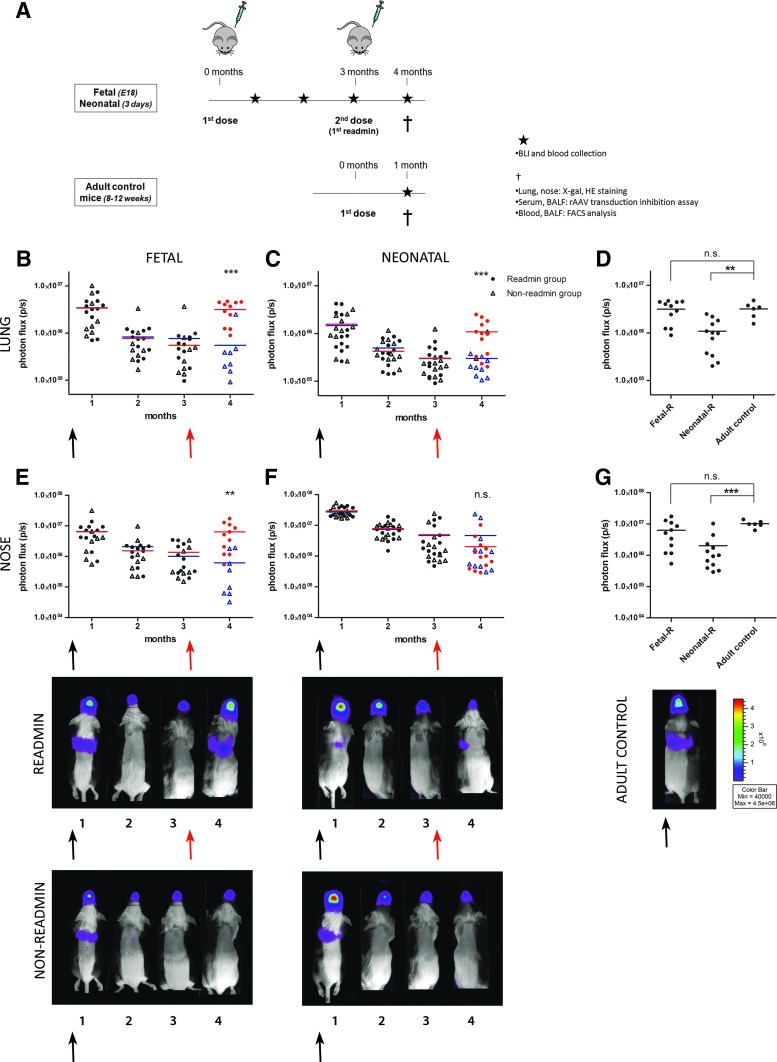
FIG. 2.
Successful readministration of rAAV2/5 after initial perinatal gene transfer. (A) Overview of the experiment designed to study the feasibility of rAAV2/5 (1.5×1010 GC/animal) readministration to murine airways after perinatal gene delivery. Firefly luciferase (fLUC) expression was visualized (photos, bottom) and quantified (B–G) over time using bioluminescence imaging (BLI). The pseudocolor scale of bioluminescent images depicts the photon flux per second, per square centimeter per steradian (p/sec/cm2/sr). Total photon flux (p/sec) was quantified over time for nose and lung. Measurements of individual animals were plotted as single values and the average BLI signal per group per time point is depicted. The black arrow depicts the first vector dose received as fetus/neonate; the red arrow indicates readministration in adult life. Red circles, BLI signal after readministration; blue triangles, non-readministration controls. (D and G) The lung and nose signals of animals that received a second vector dose after perinatal gene delivery were compared with the signal measured in adult controls who received a single vector dose, 1 month postinjection. One-way ANOVA and Tukey HSD post-hoc test, **p<0.01, ***p<0.001. Abbreviations: BALF, bronchoalveolar lavage fluid; FACS, fluorescence-activated cell sorting; Fetal-R or Neonatal-R, fetal or neonatal vector delivery and readministration at 3 months; HE, hematoxylin and eosin; n.s., not significant.