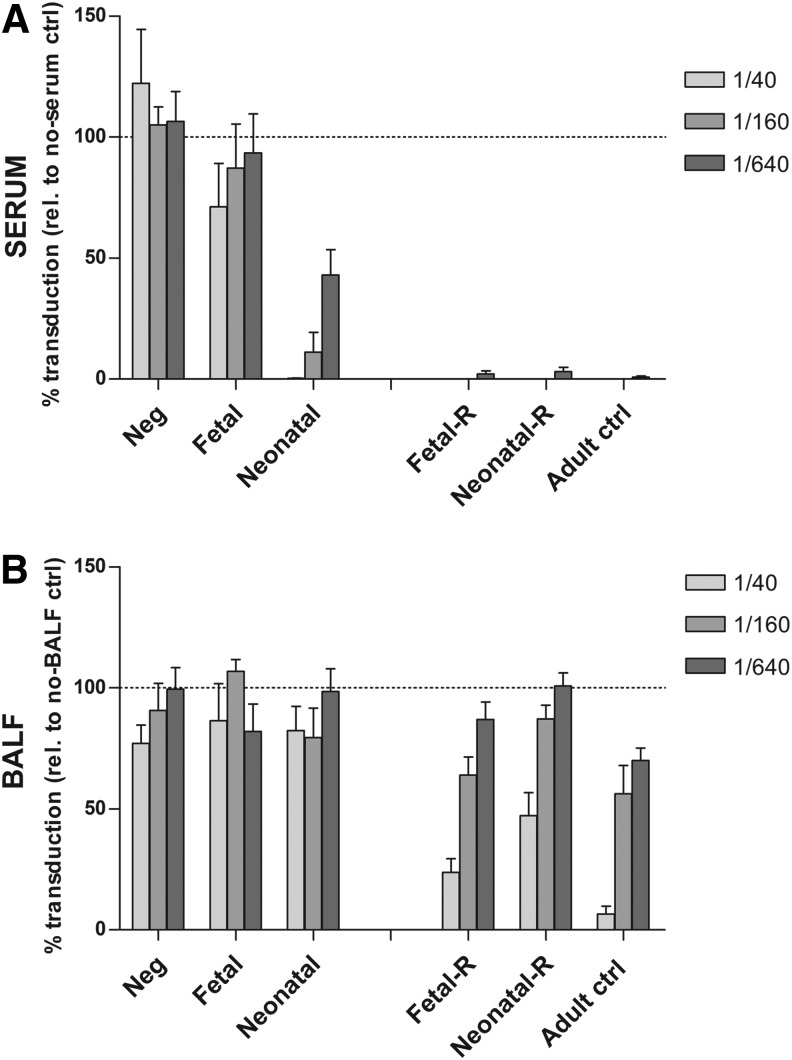
FIG. 3.
Analysis of neutralizing antibody (nAb) against the rAAV2/5 capsid after perinatal gene transfer and after readministration. An rAAV transduction inhibition assay was performed to analyze nAb against the rAAV2/5 capsid in serum and BALF. Samples were analyzed 4 months after fetal or neonatal rAAV2/5 delivery, or 1 month after readministration. Serial dilutions of serum (A) or BALF samples (B) were incubated with rAAV2/5-fLUC and tested for inhibition of transduction in HEK293T cells. Transduction was quantified by measuring relative light units (RLU) per microgram of protein and is expressed as mean percent transduction relative to no-serum or no-BALF control±SEM. The dashed line represents 100% transduction as measured by no-serum or no-BALF control. Fetal or neonatal, non-readministration controls 4 months after fetal or neonatal rAAV2/5 delivery; Fetal-R or Neonatal-R, fetal or neonatal vector delivery and readministration at 3 months, serum/BALF collection 1 month later; Adult ctrl, adult controls that received a single vector dose, serum/BALF collection 1 month postinjection.