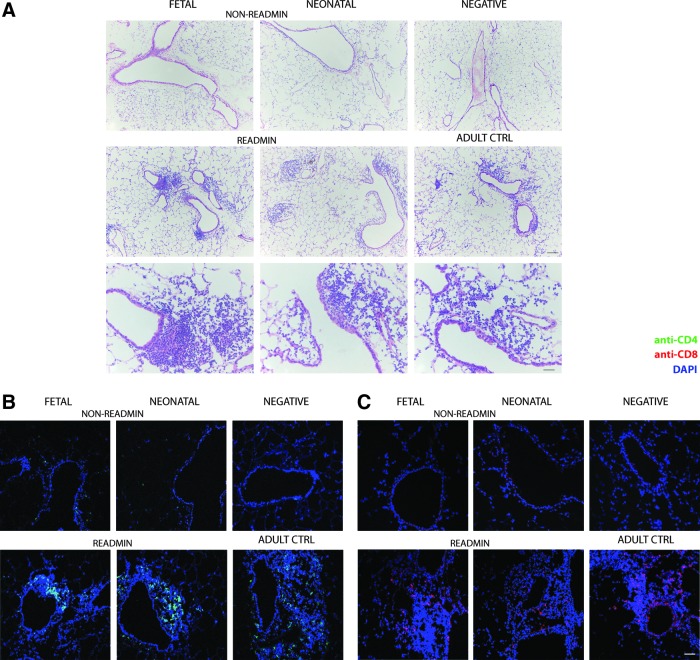
FIG. 4.
Evaluation of lung inflammation after perinatal rAAV2/5 administration or readministration in adult life. (A) Infiltration of immune cells in lung parenchyma was evaluated by hematoxylin–eosin (H&E) staining 4 months after fetal and neonatal rAAV2/5 administration (1.0×1010 GC of rAAV2/5-β-Gal per animal and 1.5×1010 GC of rAAV2/5-fLUC per animal) and 1 month after readministration at 3 months. Samples of negative and adult control mice that received a single vector dose were analyzed in parallel. Representative images at various time points depict peribronchial, peribronchiolar, and perivascular regions in which infiltration of immune cells was assessed. (B) Anti-CD4–Alexa 488 staining for identification of CD4+ T cells in lymphocytic infiltrates. (C) Anti-CD8–Alexa 555 staining for identification of CD8+ T cells in lymphocytic infiltrates. 4′,6-Diamidino-2-phenylindole (DAPI) was used for nuclear staining. Scale bars: 50 μm, except scale bar H&E bottom row: 200 μm. Abbreviations: readmin, readministration at 3 months and tissue collection at 4 months; non-readmin, non-readministered controls 4 months after perinatal gene transfer.