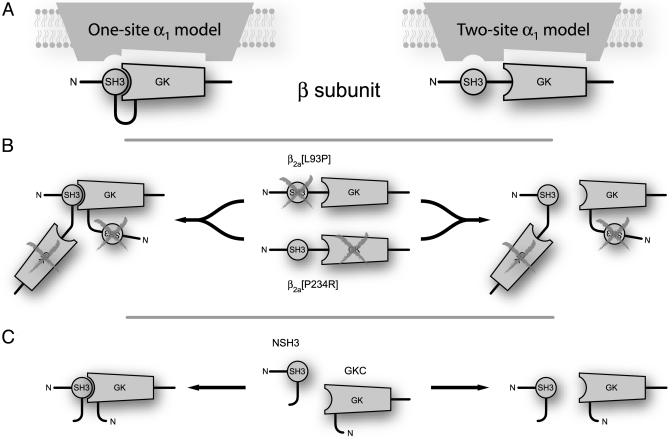
Fig. 5.
Conceptualization of β-structural determinants for modulation of α1-subunits. (A) In WT β, intramolecular SH3 and GK interaction could form a functional unit that modulates α1-subunits (one-site model; Left). By contrast, SH3 and GK domains could interact with independent binding sites on α1 (two-site model; Right). (B) SH3 and GK mutants are functionally deficient. However, trans complementation could reconstitute a functional unit (one-site model; Left) or separately provide WT SH3 and GK domains to accommodate independent binding sites (two-site model; Right). (C) Hemi-β2a-subunits could operate similarly.