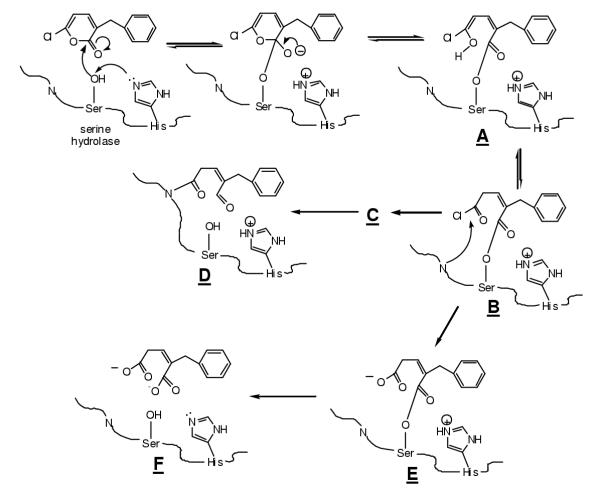
Figure 6.
Substituted 6-chloro-2-pyrones can function as (1) pseudosubstrates of serine hydrolases in which the acylenzyme intermeditate B accumulates; (2) suicide inhibitors in which formation of B is followed by attack of an active site nucleophile to form C and deacyclation to form D; (3) simple substrates in which the reactive acylchloride B hydrolyzes to acylenzyme E followed by deacylation to regenerate active enzyme F with liberation of the substituted glutaconic acid product. Alternatively, substituted 6-chloro-2-pyrones can be simple reversible inhibitors or irreversible active site inhibitors where an active site nucleophile attacks the 6-postion of the pyrone with release of chloride.