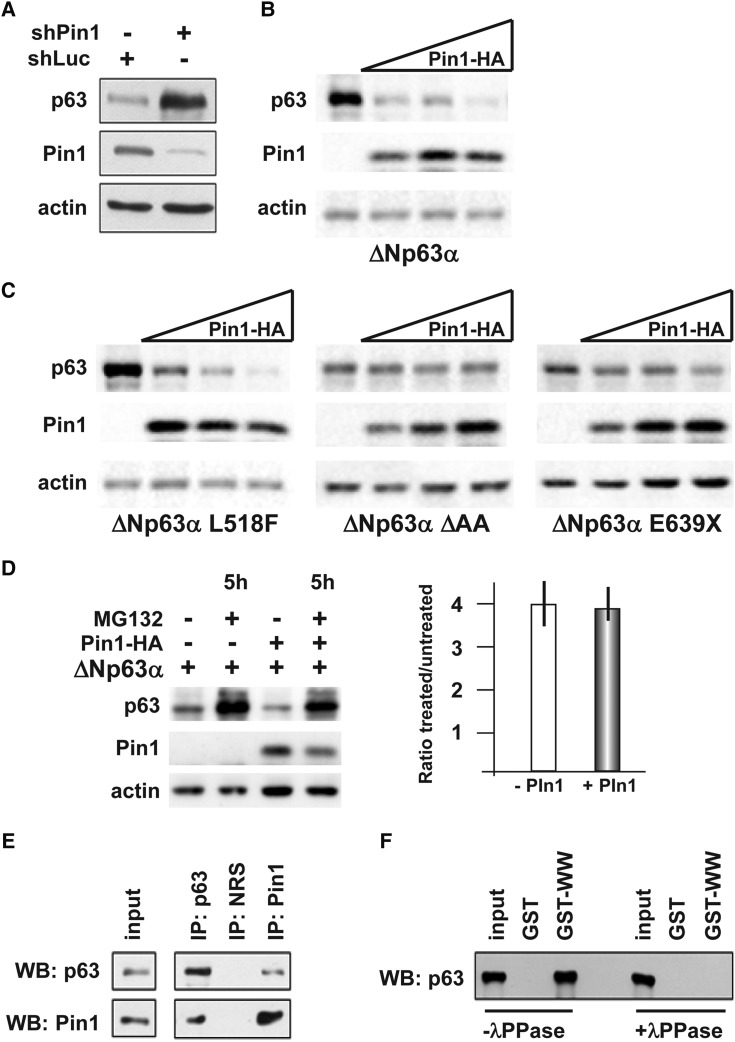
Figure 3.
The Pin1 isomerase promotes ΔNp63α degradation. (A) Western blot analyses of whole protein extracts of U2OS cells transiently co-transfected with ΔNp63α (50 ng) and an anti-Pin1 siRNA (10 pmol/cm2), or an anti-LacZ siRNA (siCtr 10 pmol/cm2) as negative control. Actin is used for loading control. (B) Western blot analyses of whole protein extracts from U2OS cells transiently co-transfected with increasing amounts (20, 40 and 80 ng) of Pin1–HA-tagged vector (indicated on top), and WT ΔNp63α (30 ng). The α-isoform is efficiently degraded by Pin1 expression, whereas the β- and the γ-isoforms are not (Supplementary Material, Fig. S1). (C) Western blot analyses of whole protein extracts from U2OS cells transiently co-transfected with increasing amounts (20, 40 and 80 ng) of Pin1–HA-tagged vector (indicated on top), and the disease-relevant mutated versions of ΔNp63α L518F (AEC associated), ΔAA (LMS associated) and E639X (SHFM associated) (30 ng each) (indicated at the bottom). Actin is used for loading control. The mutant p63 proteins linked to congenital limb malformations (LMS and SHFM) are relatively resistant against Pin1-induced degradation, compared with WT p63, whereas the mutant p63 linked to AEC, with no limb defects, is sensitive to Pin1. (D) Western blot analyses of whole protein extracts from U2OS cells, transiently co-transfected with ΔN-p63α and Pin1–HA-tagged expression vectors, and 20 h later either treated with 5 μm of the proteasome inhibitor MG132 or left untreated (DMSO only). Proteins were extracted after 5 h of treatment and assayed by western analysis; actin is used for loading control. On the right, quantification of the p63 protein level, expressed as the ratio between the treated and the untreated sample, in cell transfected (gray bar) or not transfected (open bar) with Pin1. The results show that Pin1-mediated destabilization of p63 is mainly mediated by the proteasome. (E) Western blot analyses of proteins immunoprecipitated with anti-p63 (IP p63) or anti-Pin1 (IP Pin1) antibodies, revealed using, respectively, anti-Pin1 (bottom, lanes) and anti-p63 (top panel). Input control is shown on the left. (F) Western blot analysis of GST-pull down assay done with anti-Pin1, revealed with anti-p63 antibody, in the presence (+λPPase, on the right) or absence (−λPPase, on the left) of protein phosphatase during the pull-down. The input sample is also loaded as control. While untreated samples contained p63, in the presence of IPP the p63 protein is absent.