Abstract
Anatomical left-right (L/R) handedness asymmetry in C. elegans is established in the four-cell embryo as a result of anteroposterior skewing of transverse mitotic spindles with a defined handedness. This event creates a chiral embryo and ultimately an adult body plan with fixed L/R positioning of internal organs and components of the nervous system. While this “dextral” configuration is invariant in hermaphrodites, it can be reversed by physical manipulation of the early embryo or by mutations that interfere with mitotic spindle orientation, which leads to viable, mirror-reversed (sinistral) animals. During normal development of the C. elegans male, the gonad develops on the right of the midline, with the gut bilaterally apposed on the left. However, we found that in males of the laboratory N2 strain and Hawaiian (“Hw”) wild isolate, the gut/gonad asymmetry is frequently reversed in a temperature-dependent manner, independent of normal embryonic chirality. We also observed sporadic errors in gonad migration occurring naturally during early larval stages of these and other wild strains; however, the incidence of such errors does not correlate with the frequency of L/R gut/gonad reversals in these strains. Analysis of N2/Hw hybrids and recombinant inbred advanced intercross lines (RIAILs) indicate that the L/R organ reversals are likely to result from recessively acting variations in multiple genes. Thus, unlike the highly reproducible L/R asymmetries of most structures in hermaphrodites, the L/R asymmetry of the male C. elegans body plan is less rigidly determined and subject to natural variation that is influenced by a multiplicity of genes.
Keywords: wild isolate, gonad migration, RIAIL, genetic heterogeneity, natural variation, gpa-16
While many animals are superficially bilaterally symmetric, the arrangements of internal organs, as well as structures in the nervous system, are often left-right (L/R) asymmetric, generally with a defined and reproducible handedness. In rare individuals, this normal handedness is completely reversed, as in the human condition situs inversus totalis (Afzelius, 1976). Such individuals show mirror-reversed arrangement of visceral organs but generally suffer no overt consequences. Ontogenic defects can also result in partial L/R reversals in the anatomical arrangements of organs, as in situs inversus incompletus, or in a deranged organization with no discernible handedness (heterotaxia; Levin, 2005). Such incomplete disorders of laterality can result in dysfunctional organ systems. The genetic and molecular bases for such partial L/R reversals are not well understood.
The internal anatomy of the nematode C. elegans is highly L/R asymmetric and most bilateral asymmetries occur with a defined handedness. While bilateral asymmetry in the animal is evident in L/R functional differences between otherwise bilaterally symmetric neurons (Hobert et al., 2002), it is most apparent in the bilateral arrangement of the intestine and gonad, which run longitudinally along most of the length of the worm. This prominent L/R anatomical asymmetry traces back to the early embryo, which becomes L/R asymmetric in cellular arrangements immediately after the establishment of the anterior-posterior (A/P) and dorsal-ventral axes, created during the first two rounds of cell division. This L/R embryonic chirality is established when the two descendants (called ABa and ABp) of the anterior daughter of the zygote, AB, divide along the L/R axis. Though initially symmetrically arranged, the mitotic spindles in these dividing cells subsequently skew along the A/P axis with a defined handedness. This event results in the bilaterally asymmetric arrangement of cells in the 6-cell embryo, subsequently leading to L/R differences in cellular interactions, primarily via Notch signalling (Priess, 2005). The bilaterally asymmetric interactions create striking bilateral differences in cell lineages, identities, and positions that persist into adulthood (Poole and Hobert, 2006; Priess and Thomson, 1987; Wood, 1991). These events are virtually invariant, resulting in adults with uniform (arbitrarily defined as “dextral”) handedness in the arrangement of organs and the nervous system. However, the entire L/R anatomical handedness can be reversed (i.e., situs inversus totalis) by micromanipulation of the skewing spindles (Wood, 1991), cold treatment (Wood et al., 1996), eggshell removal (Wood and Kershaw, 1991), or mutations that alter mitotic spindle orientation in the early embryo (Bergmann et al., 2003). Such mirror-reversed, or “sinistral” animals are viable and healthy.
While C. elegans hermaphrodites invariably show a dextral arrangement of organs (i.e., no reversals observed in n>15,000 animals; Bergmann et al., 2003; Wood, 1991; our unpublished results), we have found that males of some wild isolates, and of the commonly used “N2” laboratory strain under particular conditions, exhibit situs inversus incompletus, with L/R reversals in bilateral arrangement of the major organs, the gonad and gut, independent of the L/R laterality of the whole animal (Fig. 1). During gonad development in the hermaphrodite, the two distal tip cells lead the longitudinal migration of the gonad in opposite directions from the midbody. As one distal tip cell moves anteriorly on the right, the other moves posteriorly on the left side, creating a two-armed, S-shaped gonad, with the vulva at the midpoint. The intestine occupies the contralateral position. The male gonad consists of only a single arm, which is formed by migration led by the linker cell anteriorly from the midbody on the right side of the animal. The linker cell and developing gonad then turns dorsally and migrates posteriorly forming a J-shaped organ running along the right side of the animal, resulting in a left-side orientation of the intestine.
Figure 1. Dextral and sinistral gut/gonad orientation.
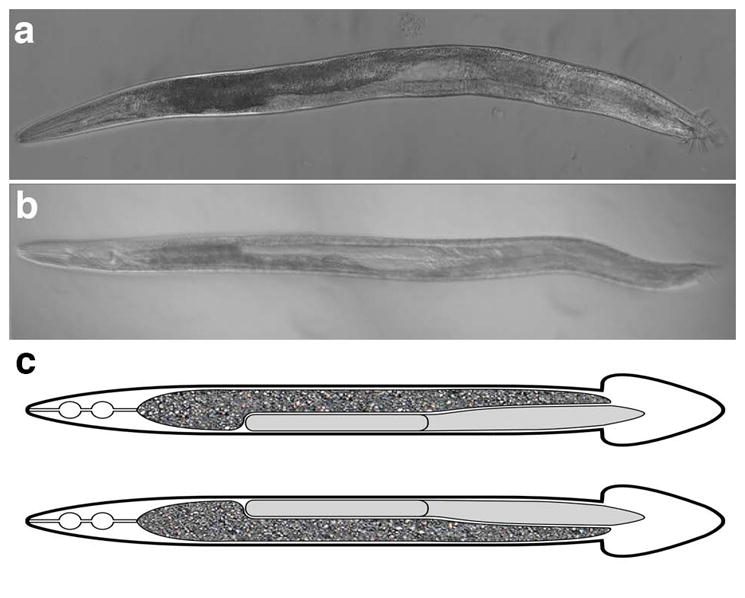
Ventral view of adult males is shown, with anterior to left. a) Bright field image of worm with normal (dextral) gut/gonad orientation. b) Bright field image of animal from the Hawaiian (Hw) strain, CB4856, with reversed gut/gonad orientation. c) Cartoon of normal worm (top) and reversed worm (bottom). The gut is indicated by the granular texture, with the gonad in gray.
From the N2 laboratory strain grown at 20°C, we found rare (2/522; 0.4%) examples of males with reversed gut/gonad asymmetry (Figs. 1 and 3): while the overall shape of the gonad and gut were unchanged, these organs show an opposite orientation, with the gonad on the left and gut on the right (Fig. 1b). The frequency of such animals was dramatically elevated in the same strain cultured at 25°C: 5.4% (25/466, p < 0.05) of males showed gut/gonad reversals. The higher temperature was not essential for frequent reversals: we found that the Hawaiian (“Hw”) wild isolate (strain CB4856) often (5.8%, 49/846) shows reversed gut/gonad asymmetry at 20°C, significantly more than N2 (p < 0.05) and a somewhat increased proportion of reversals (8.1%, 55/677, p = 0.08) at 25°C. We are unaware of any studies reporting natural reversal of hermaphrodites at 25°C.
Figure 3. Gonad migration errors.
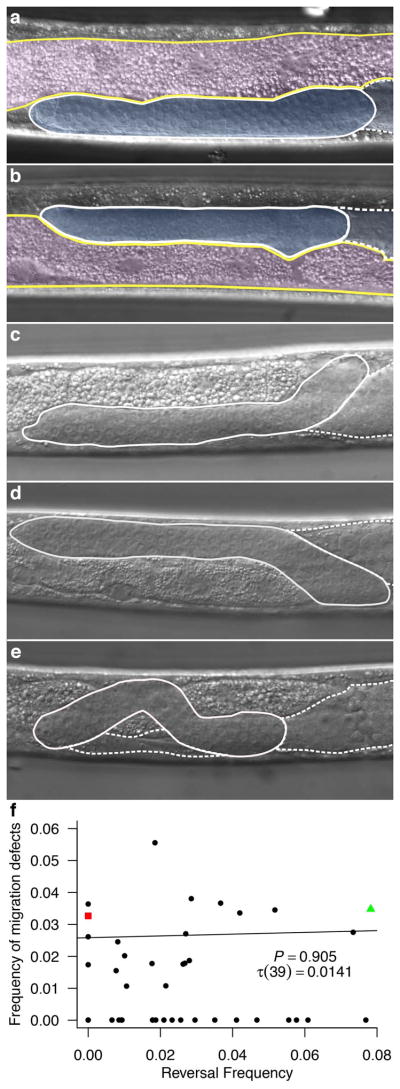
a–e) Nomarksi microscopic images of male gonads in midbody region. a) N2 with normal gonad. b) CB4856 (Hw) with reversed gut/gonad asymmetry. c) Wild isolate (JU397) normal gonad with migration error where gonad begins on incorrectly on the left and corrects to grow on the right. d) Wild isolate (JU1172) with reversed gut/gonad orientation and migration error where the gonad begins correctly on the right and then mis-migrates to grow on the left. e) Wild isolate (JU1213) with normal gonad orientation and migration error with multiple midline crossing errors. False color in a–b gut is outlined in yellow and highlighted with pink. The gonad is outlined with white and highlighted with blue. c–e) Gonad is outlined in white; dashed white outline indicates the area of gonad that lies medially to the posterior end of the gonad. f) No significant correlation (n = 93 to 245, Kendall’s correlation τ (39) = 0.01, p = 0.91) between worms with a migration defect and worms with gut-gonad reversal. Wild isolates, black circles; N2, red square; Hw, green triangle. Strains used are listed in Materials and Methods. All images are presented with a ventral view and anterior to left. All experiments were carried out at 20°C.
We sought to investigate whether the L/R reversal in gut/gonad asymmetry was the result of complete or partial reversal in anatomical handedness. In hermaphrodites, L/R reversal in the arrangement of the gut and gonad is indicative of a complete mirror-image reversal of the entire anatomy of the animal and has been used as the salient marker of reversal in embryonic chirality (Bergmann et al., 2003; Wood, 1991; Wood et al., 1996; Wood and Kershaw, 1991). Another marker of such situs inversus totalis events is reversal in gene expression of the ASE gustatory neuron pair (ASEL and ASER). While these neurons are morphologically symmetric, they are functionally distinct as a result of differential expression of GCY family chemoreceptors (Poole and Hobert, 2006; Yu et al., 1997). ASER, on the right, invariably expresses gcy-5 (Fig. 2a–b, d); however, its contralateral homologue, ASEL, never expresses this gene in dextral animals (Yu et al., 1997). In hermaphrodites with situs inversus totalis, resulting from a gpa-16 mutation, gcy-5 is expressed in the left neuron and not the right (Fig. 2c; Bergmann et al., 2003; Poole and Hobert, 2006), reflecting reversal in L/R specification in the nervous system. We observed that, as expected, 100% (13/13) of reversed gpa-16 males similarly show reversal in the L/R asymmetric expression of gcy-5 (Fig. 2c). In contrast, we found that gcy-5 expression is retained in the right neuron of the ASER/L pair in 100% (8/8) of males from the N2 laboratory reference strain in which the bilateral orientation of the gut and gonad are reversed (Fig. 2b), suggesting that gut-gonad reversal occurs independently of the event that establishes the overall L/R handedness in the embryo. Consistent with these findings, we observed no example of reversals in early embryonic chirality under conditions in which gut/gonad reversals were frequently observed. These results suggest that the gut/gonad reversal occurs independently from the mechanism that establishes the overall embryonic chirality and L/R asymmetry of other structures, to our knowledge the first example of naturally occurring situs inversus incompletus in C. elegans.
Figure 2. Reversed gut/gonad orientation is independent of L/R asymmetric expression of gcy-5 in the ASER neuron.
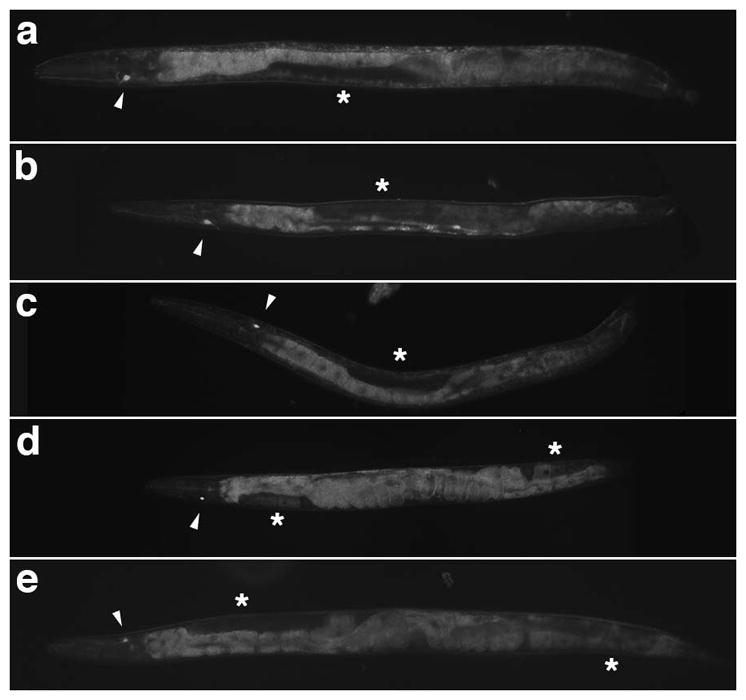
a) Normal (dextral) male cultured at 20°C expressing gcy-5p::GFP on the right. b) Male cultured at 25°C with reversed gut/gonad asymmetry expressing gcy-5p::GFP. Note that gcy-5 is expressed on the right as with the animal with normal gut/gonad orientation. c) gpa-16(it143) I; gcy-5p::GFP V male cultured at 25°C with situs inversus totalis. gcy-5 is expressed on the left, owing to the complete mirror-image anatomical handedness. d) Normal (dextral) hermaphrodite cultured at 20°C expressing gcy-5p::GFP on the right. e) gpa-16(it143) I; gcy-5p::GFP V hermaphrodite grown at 25°C showing sinistral anatomical handedness and gcy-5 expression on the left. All animals are presented with ventral side up and anterior to left. Arrowhead indicates position of the gcy-5-expressing neuron. Asterisk indicates position of the gonad.
While defects in gonad migration are rarely observed during normal C. elegans male development, many mutations have been identified that cause gonad migration defects, primarily during the L2–L4 larval stages, when the migrating linker cell fails to migrate dorsally, to return ventrally, or to migrate posteriorly (Hedgecock et al., 1990; Kato and Sternberg, 2009; Vogel and Hedgecock, 2001). However, defects in L/R laterality of the gonad have not been reported with these mutations. It is conceivable that the gut/gonad reversals we observed might result from aberrant gonad migration at early stages. Indeed, in N2, Hw, and a number of other wild isolates (Andersen et al., 2012) we observed frequent errors in gonad migration across the L/R axis during the L1 linker cell migration. We found three types of errors: (1) the linker cell begins migration inappropriately on the left side and then makes an immediate turn to the right side, where it completes migration (Fig. 3c), (2) the linker cell shows the opposite behavior, by starting migration on the right and then immediately switching to the left, where it completes its migration (Fig. 3d), or (3) the linker cell begins migration on the right side, then meanders multiple times across the midline, and finally completes migration on the right side of the animal (Fig. 3e). We compared the frequency of these migration errors (ranging from zero to 5.6%) with the frequency of gut/gonad reversals (ranging from zero to 7.8%) across the 40 wild isolate strains examined and found that there was no significant correlation between the frequency of these two behaviors (Kendall’s τ (39) = 0.0141, p = 0.91, Fig. 3f). Thus, the reversal in gut/gonad L/R asymmetry cannot be explained primarily by the same mechanism that leads to these early gonad migration defects.
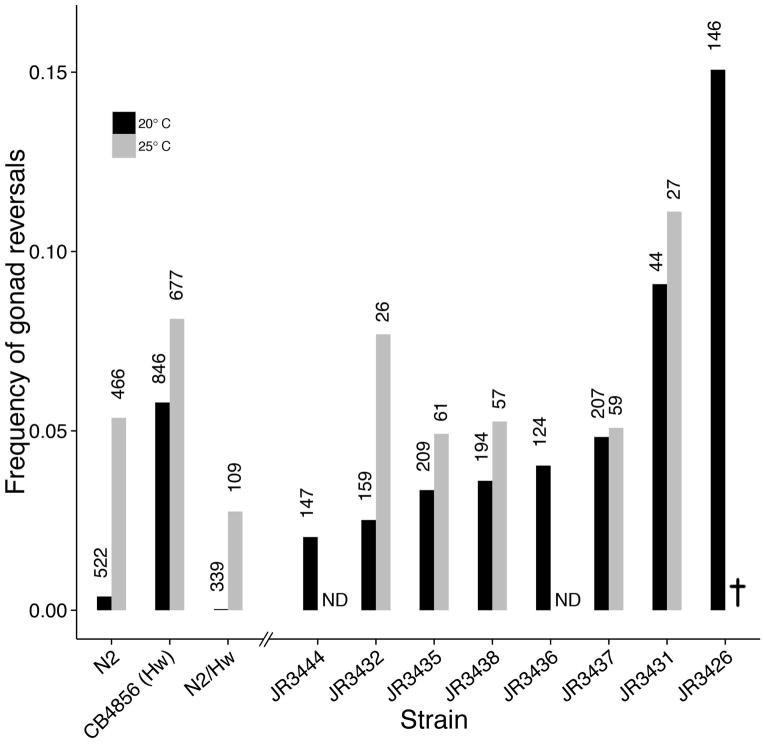
Genetic observations suggest that multiple genes may influence the extent of L/R reversals in gut/gonad asymmetry, which would be consistent with the substantial variation observed. As an initial assessment of the genetic complexity underlying this effect, we analyzed F1 hybrids from an N2 x Hw cross. At 20°C, while the Hw strain showed 5.8% reversals (see above), the N2 x Hw hybrids showed no reversals (n = 339), which was the same as the very low reversal frequency (0.4%) seen for the N2 strain under these conditions (p = 0.52, Fig. 4). These results suggest that the frequent reversal trait of the Hw strain is fully recessive. Further, we found that while both N2 and Hw showed > 5% reversals at 25°C, only 2.8% (n = 109) of the F1 hybrids were reversed under these conditions, lower than either parental strain alone (5.4%, p = 0.33 and 8.1%, p < 0.05 for N2 and Hw respectively). Thus, it is conceivable that there are distinct, recessive polymorphisms in the N2 and Hw strains that perturb the dominant dextral gut/gonad asymmetry and that complement each other, resulting in suppression of the trait in the F1 generation.
Figure 4. Gut-gonad reversal frequency of male worms in two wild strains, F1 hybrids, and recombinant inbred advanced intercross lines (RIAILs) at two temperatures.
Black bars indicate animals grown at 20°C, gray bars, animals grown at 25°C. Number of animals scored are indicated above each bar. ND, not determined. JR3426 is inviable at 25°C. See text for further information.
To further assess the genetic complexity influencing the extent of L/R gut/gonad reversals, we took advantage of a set of recombinant inbred advanced intercross lines (RIAILs) generated between the N2 and Hw strains (Rockman and Kruglyak, 2009). The intercrossing strategy used to generate RIAILs allows for many recombination events per chromosome, allowing for fine-scale mapping of traits. Our analyses suggested genetic heterogeneity underlying the trait, with a spectrum of reversal frequencies observed. While some of the RIAILs showed rates of reversal that were intermediate between the two parental strains, we observed reversal frequencies substantially higher than that of either parent (as high as 15.1% at 20°C; p < 0.05 for both parental strains, Fig. 4), revealing transgressive segregation (Rieseberg et al., 1999) of the trait. In all cases, the frequency of reversals was increased at 25°C; however, we were unable to score the most strongly affected RIAIL (JR3426) owing to fully penetrant lethality at 25°C. These findings suggest that multiple genes contribute to the rate of L/R reversal in gut/gonad asymmetry and imply that genetic variations in both parents lead to non-uniform handedness in the arrangement of these organs.
The highly stereotyped cell lineage patterns, cell positioning, cellular differentiation, and anatomy are salient features of C. elegans development (Kimble and Hirsh, 1979; Priess and Thomson, 1987; Sulston et al., 1980; Sulston and Horvitz, 1977). However, more recently, it has become clear that there is substantial variation in cell position, cell cycle timing, and other developmental traits when observed at a sufficiently fine-grained level (Bao et al., 2008; Braendle and Felix, 2008; Dolgin et al., 2008; Liu et al., 2009; Moore et al., 2013; Schnabel et al., 1997) Nonetheless, the overall anatomical handedness has been considered a highly reproducible, determinate characteristic of the animal. We have shown here that the L/R handedness in the arrangement of the largest organs in the animal is naturally subject to unexpected variation in response to environmental conditions and genetic differences between wild isolates. As the gut and gonad comprise much of the structure of the animal, the situs inversus incompletus that we have observed is the largest natural variation in terms of mass of tissue that has been observed in C. elegans.
While whole animal L/R reversals in worms can be caused by aberrant spindle orientations during the early embryonic cell divisions (of ABa and ABp), the novel gut/gonad reversal effect that we have discovered points to a second symmetry-breaking event that asymmetrically positions the major organs during later development. Thus, the apparent strong selection for defined anatomical handedness in the worm may be somewhat relaxed in males of the hermaphroditic species, which are non-essential. Further the greater simplicity in anatomical packing of the single-arm male J-shaped gonad compared to the more complex hermaphrodite gonad anatomy may lead to greater plasticity in organ arrangement. It is of interest to note that another L/R trait in the male of C. elegans and other nematode species, the direction of turning during mating is also established independently of embryonic chirality (Downes et al., 2012).
It is conceivable that the twist of the intestine that is established during embryogenesis with a defined L/R handedness in response to a LIN-12/Notch-dependent signal (Hermann et al., 2000) influences the L/R orientation of the gut/gonad asymmetry. In such an event, the natural variation in this asymmetry might result from alterations in the Notch signalling event and gut twist, though the role of gut twist on L/R orientation of the gut and gonad has not been investigated. Regardless, the natural variation we have observed between wild strains provides genetic access to the molecular controls over organ handedness asymmetry in this animal.
Materials and Methods
General maintenance of C. elegans and strains used
Except where noted, worms were maintained as described by S. Brenner (Brenner, 1974) at 20°C and scored at room temperature. All strains were provided from laboratory stocks, the Caenorhabditis Genetics Center, or the Kruglyak laboratory (RIAILS and some wild isolates). The strains used in this study are listed in Table 1.
Table 1.
C. elegans strains used.
| Strain | Genotype |
|---|---|
| N2 | |
| OH3192 | ntIs1 [gcy-5p::GFP + lin-15(+)] V |
| BW1809 | gpa-16(it143) I; him-5(e1490) V |
| JR3653 | gpa-16(it143) I; ntIs1 [gcy-5p::GFP + lin- 15(+)] V |
| JR3426 | RIAIL Line 100 |
| JR3431 | RIAIL Line 127 |
| JR3432 | RIAIL Line 133 |
| JR3435 | RIAIL Line 157 |
| JR3436 | RIAIL Line 160 |
| JR3437 | RIAIL Line 163 |
| JR3438 | RIAIL Line 169 |
| JR3444 | RIAIL Line 190 |
| Wild Isolates: CB4852, CB4854, CB4856 (Hw), CB4857, CB4858, CB4932, CX11264, CX11271, CX11285, CX11307, CX1134, DL238, ED3011, ED3012, ED3017, ED3046, ED3048, EG4347, EG4349, JU311, JU322, JU323, JU345, JU360, JU363, JU397*, JU751, JU774, JU830, JU1088, JU1172, JU1213*, JU1400, JU1409, JU1440, JU1652, JU1896, LSJ1, MY14, PB306, PS2025, QX1233 | |
Migration data were not collected for these strains, and therefore they were not included in the correlation analysis in Figure 3f.
Generation of male worms
To obtain male strains, 20–30 L4 hermaphrodites were picked into a 7% ethanol solution in Eppendorf tubes and rotated for 1h (modified from Lyons and Hecht, 1997). Worms were subsequently sedimented by centrifugation at 2000 rpm for 10s. Pelleted worms were plated onto fresh NGM plates seeded with OP50 and incubated at 20°C. F1 male progeny were collected after several days and used to create male stocks.
Scoring of gut and gonad position and linker cell migration
Males were collected, anesthetized in 5 mM levamisol and mounted ventral side up on a 5% agar pad with a platinum pick and eyelash tool. Mounted worms were then imaged at 20x or 63x magnification on a Zeiss AxioSkop 2.
Statistical Analyses
Data analyses were performed with R Statistical Software, version 2.14.1. Kendall’s τ was used for correlation analyses instead of Pearson’s, as the reversal frequencies of the worm strains were not normally distributed. Fisher’s exact test was used to test pairwise comparisons of reversal frequencies between different strains at each temperature (20°C and 25°C) and between temperatures for each strain.
Acknowledgments
We would like to thank Derek Tan and Adam Crowe for help with data collection and figure creation; the Kruglyak laboratory for sharing RIAILs and wild isolate strains. Some strains were provided by the CGC, which is funded by the NIH Office of Research Infrastructure Programs (P40 OD010440). This work was funded by an NIH Ruth L. Kirschstein National Research Service Award and a Harvey Karp Discovery Award to BB, and by grants from the National Institute of General Medical Sciences (1R21GM094649) and the National Institute of Child Health and Human Development (1R01 HD062922) to JHR.
Literature Cited
- Afzelius BA. A human syndrome caused by immotile cilia. Science. 1976;193:317–319. doi: 10.1126/science.1084576. [DOI] [PubMed] [Google Scholar]
- Andersen EC, Gerke JP, Shapiro JA, Crissman JR, Ghosh R, Bloom JS, Felix MA, Kruglyak L. Chromosome-scale selective sweeps shape Caenorhabditis elegans genomic diversity. Nat Genet. 2012;44:285–290. doi: 10.1038/ng.1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bao ZR, Zhao ZY, Boyle TJ, Murray JI, Waterston RH. Control of cell cycle timing during C. elegans embryogenesis. Dev Biol. 2008;318:65–72. doi: 10.1016/j.ydbio.2008.02.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergmann DC, Lee M, Robertson B, Tsou MF, Rose LS, Wood WB. Embryonic handedness choice in C. elegans involves the Galpha protein GPA-16. Development. 2003;130:5731–5740. doi: 10.1242/dev.00839. [DOI] [PubMed] [Google Scholar]
- Braendle C, Felix MA. Plasticity and errors of a robust developmental system in different environments. Dev Cell. 2008;15:714–724. doi: 10.1016/j.devcel.2008.09.011. [DOI] [PubMed] [Google Scholar]
- Brenner S. The genetics of Caenorhabditis elegans. Genetics. 1974;77:71–94. doi: 10.1093/genetics/77.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolgin ES, Felix MA, Cutter AD. Hakuna Nematoda: genetic and phenotypic diversity in African isolates of Caenorhabditis elegans and C. briggsae. Heredity (Edinb) 2008;100:304–315. doi: 10.1038/sj.hdy.6801079. [DOI] [PubMed] [Google Scholar]
- Downes JC, Birsoy B, Chipman KC, Rothman JH. Handedness of a motor program in C. elegans is independent of left-right body asymmetry. PLoS One. 2012;7:e52138. doi: 10.1371/journal.pone.0052138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedgecock EM, Culotti JG, Hall DH. The unc-5, unc-6, and unc-40 genes guide circumferential migrations of pioneer axons and mesodermal cells on the epidermis in C. elegans. Neuron. 1990;4:61–85. doi: 10.1016/0896-6273(90)90444-k. [DOI] [PubMed] [Google Scholar]
- Hermann GJ, Leung B, Priess JR. Left-right asymmetry in C. elegans intestine organogenesis involves a LIN-12/Notch signaling pathway. Development. 2000;127:3429–3440. doi: 10.1242/dev.127.16.3429. [DOI] [PubMed] [Google Scholar]
- Hobert O, Johnston RJ, Jr, Chang S. Left-right asymmetry in the nervous system: the Caenorhabditis elegans model. Nat Rev Neurosci. 2002;3:629–640. doi: 10.1038/nrn897. [DOI] [PubMed] [Google Scholar]
- Kato M, Sternberg PW. The C. elegans tailless/Tlx homolog nhr-67 regulates a stage-specific program of linker cell migration in male gonadogenesis. Development. 2009;136:3907–3915. doi: 10.1242/dev.035477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimble J, Hirsh D. The postembryonic cell lineages of the hermaphrodite and male gonads in Caenorhabditis elegans. Dev Biol. 1979;70:396–417. doi: 10.1016/0012-1606(79)90035-6. [DOI] [PubMed] [Google Scholar]
- Levin M. Left-right asymmetry in embryonic development: a comprehensive review. Mech Dev. 2005;122:3–25. doi: 10.1016/j.mod.2004.08.006. [DOI] [PubMed] [Google Scholar]
- Liu X, Long FH, Peng HC, Aerni SJ, Jiang M, Sanchez-Blanco A, Murray JI, Preston E, Mericle B, Batzoglou S, Myers EW, Kim SK. Analysis of Cell Fate from Single-Cell Gene Expression Profiles in C. elegans. Cell. 2009;139:623–633. doi: 10.1016/j.cell.2009.08.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyons LC, Hecht RM. Acute Ethanol Exposure Induces Nondisjunction of the X Chromosome During Spermatogenesis. Worm Breeder’s Gazette. 1997;14:52. [Google Scholar]
- Moore JL, Du Z, Bao Z. Systematic quantification of developmental phenotypes at single-cell resolution during embryogenesis. Development. 2013;140:3266–3274. doi: 10.1242/dev.096040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poole RJ, Hobert O. Early Embryonic Programming of Neuronal Left/Right Asymmetry in C. elegans. Current Biology. 2006;16:2279–2292. doi: 10.1016/j.cub.2006.09.041. [DOI] [PubMed] [Google Scholar]
- Priess JR. WormBook. 2005. Notch signaling in the C. elegans embryo; pp. 1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Priess JR, Thomson JN. Cellular interactions in early C. elegans embryos. Cell. 1987;48:241–250. doi: 10.1016/0092-8674(87)90427-2. [DOI] [PubMed] [Google Scholar]
- Rieseberg LH, Archer MA, Wayne RK. Transgressive segregation, adaptation and speciation. Heredity (Edinb) 1999;83 (Pt 4):363–372. doi: 10.1038/sj.hdy.6886170. [DOI] [PubMed] [Google Scholar]
- Rockman MV, Kruglyak L. Recombinational landscape and population genomics of Caenorhabditis elegans. PLoS Genet. 2009;5:e1000419. doi: 10.1371/journal.pgen.1000419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnabel R, Hutter H, Moerman D, Schnabel H. Assessing normal embryogenesis in Caenorhabditis elegans using a 4D microscope: Variability of development and regional specification. Dev Biol. 1997;184:234–265. doi: 10.1006/dbio.1997.8509. [DOI] [PubMed] [Google Scholar]
- Sulston JE, Albertson DG, Thomson JN. The Caenorhabditis elegans male: postembryonic development of nongonadal structures. Dev Biol. 1980;78:542–576. doi: 10.1016/0012-1606(80)90352-8. [DOI] [PubMed] [Google Scholar]
- Sulston JE, Horvitz HR. Post-embryonic cell lineages of the nematode, Caenorhabditis elegans. Dev Biol. 1977;56:110–156. doi: 10.1016/0012-1606(77)90158-0. [DOI] [PubMed] [Google Scholar]
- Vogel BE, Hedgecock EM. Hemicentin, a conserved extracellular member of the immunoglobulin superfamily, organizes epithelial and other cell attachments into oriented line-shaped junctions. Development. 2001;128:883–894. doi: 10.1242/dev.128.6.883. [DOI] [PubMed] [Google Scholar]
- Wood WB. Evidence from reversal of handedness in C. elegans embryos for early cell interactions determining cell fates. Nature. 1991;349:536–538. doi: 10.1038/349536a0. [DOI] [PubMed] [Google Scholar]
- Wood WB, Bergmann D, Florance A. Maternal effect of low temperature on handedness determination in C. elegans embryos. Dev Genet. 1996;19:222–230. doi: 10.1002/(SICI)1520-6408(1996)19:3<222::AID-DVG5>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- Wood WB, Kershaw D. In: Handed asymmetry, handedness reversal and mechanisms of cell fate determination in nematode embryos. Bock GR, Marsh J, editors. Chichester: J. Wiley; 1991. pp. 143–164. [DOI] [PubMed] [Google Scholar]
- Yu S, Avery L, Baude E, Garbers DL. Guanylyl cyclase expression in specific sensory neurons: a new family of chemosensory receptors. Proc Natl Acad Sci U S A. 1997;94:3384–3387. doi: 10.1073/pnas.94.7.3384. [DOI] [PMC free article] [PubMed] [Google Scholar]