Figure 1.
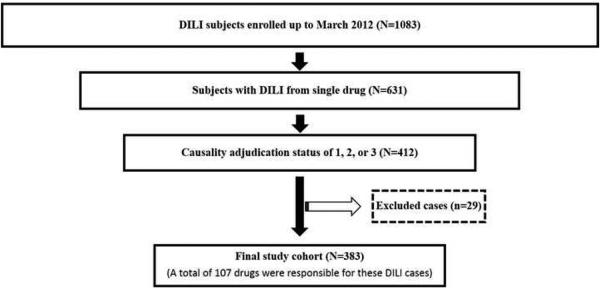
Flowchart describing the selection of the study population. A total of 1083 subjects with drug induced liver injury (DILI) were enrolled up to March 2012. Among these, 631 subjects had DILI from a single drug. After causality adjudication, there were 412 subjects with adjudication status of definite, highly likely, and probable (1, 2 or 3). An additional 29 cases were excluded due to unknown dosage, drug start/stop date or mode of administration. The final study cohort consisted of 383 DILI cases resulting from 107 different drugs satisfied the study inclusion criteria: (a) DILI occurring from a single agent, (b) administered orally, (c) known daily dosage, and (d) causality of 1, 2 or 3.
