Fig. 2.
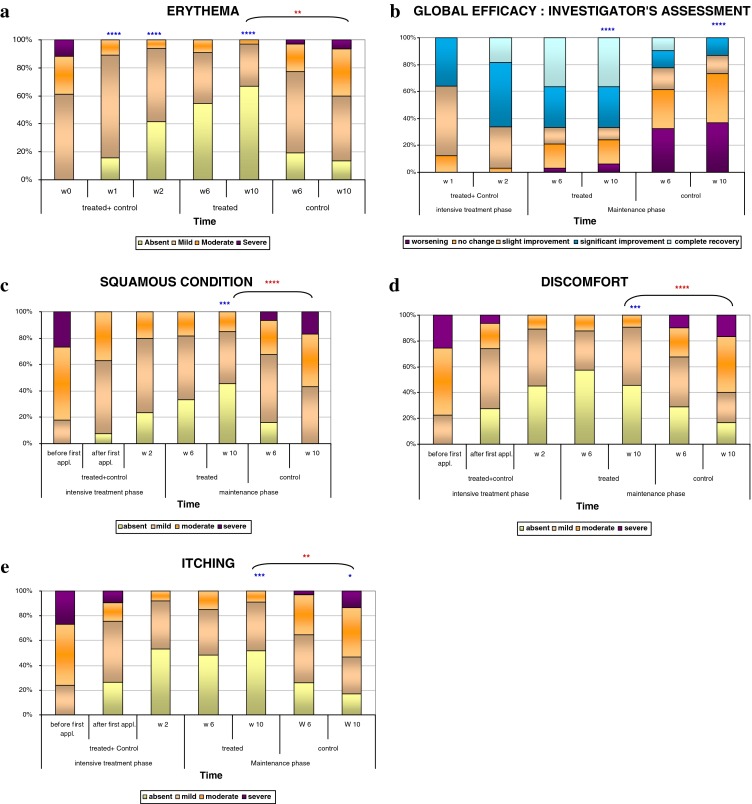
a–e Evolution of clinical criteria during the intensive treatment phase (W0 to W2) in the global study population and during the maintenance phase (W2 to W10) in control and treated patients (a erythema, b global efficacy, c self-assessment of scaling, d self-assessment of discomfort, e self-assessment of itching). For the intensive treatment phase, a paired Student’s t test or a Wilcoxon’s signed rank test, depending on normality of distributions, was used on changes between W0 and W1 and W2, respectively. For the maintenance phase, a Wilcoxon’s signed rank test was used on changes between W2 and W6 and W10, respectively. *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001, in blue: intragroup versus W0, in red: intergroup W10/W0
