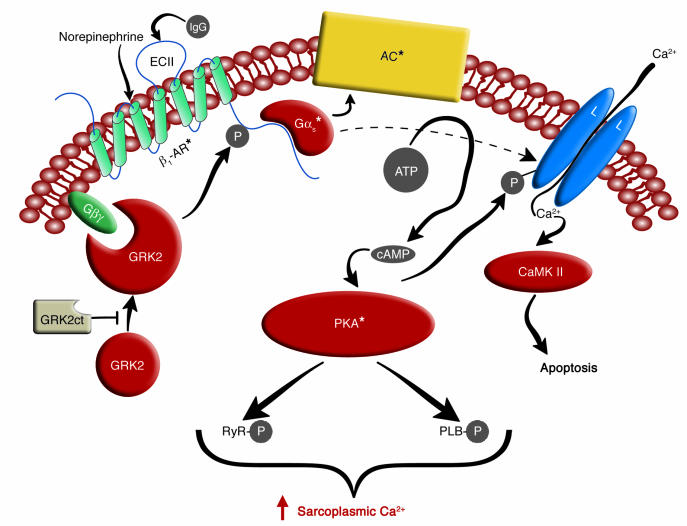
Figure 1.
Schema for β1-AR–mediated cardiomyocyte stimulation and β1-AR desensitization. The seven-membrane-spanning β1-AR is stimulated by the physiologic agonist norepinephrine or by IgG specific for the receptor’s second extracellular loop (ECII) (in CHF subjects). The stimulated β1-AR then activates the heterotrimeric Gs, which dissociates into its Gαs* and Gβγ substituents. The Gαs* activates both adenylyl cyclase (AC), which catalyzes cAMP formation, and the L-type calcium channel (L), which then permits Ca2+ to enter the cardiomyocyte. This Ca2+ can both augment contractility and, on a slower time scale, promote cardiomyocyte apoptosis by activating Ca2+/calmodulin kinase II (CaMK II). The cAMP produced by adenylyl cyclase activates PKA, which subsequently phosphorylates (P) numerous substrates important to sarcoplasmic [Ca2+] regulation: the L-type Ca2+ channel, the ryanodine receptor (RyR), and phospholamban (PLB). The net effect of this activity in the short term is to augment sarcoplasmic [Ca2+] and contractility. (In the long term, this activity engenders cardiomyocyte toxicity.) The activated β1-AR is desensitized when it is phosphorylated by PKA (not shown) and by GRK2, the cellular expression of which increases with chronic β1-AR stimulation. Translocation of GRK2 from the sarcoplasm to the sarcolemma requires Gβγ subunits, and this translocation is inhibited when GRK2ct is expressed heterologously in cardiomyocytes. Asterisks denote activated proteins.