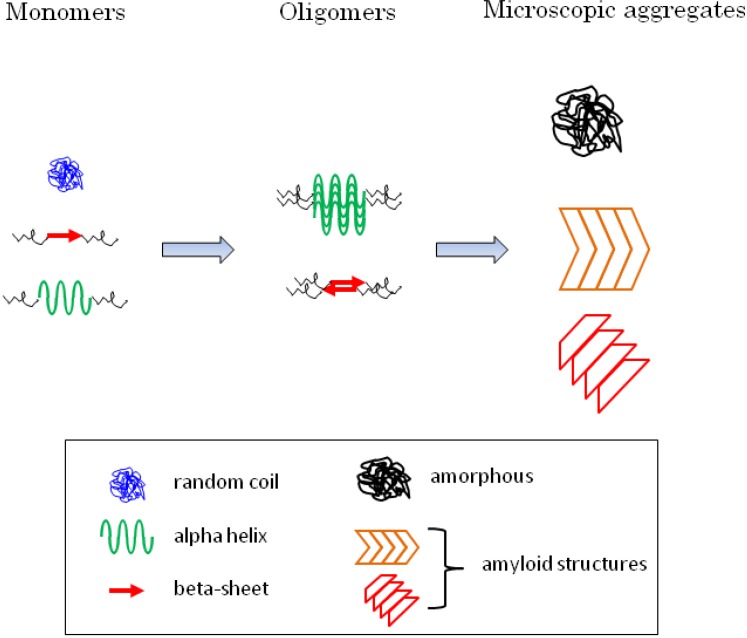
Figure 1.
Monomeric and aggregated expanded huntingtin consist of structurally heterogeneous particles. The polyQ in soluble monomeric fragments of expanded huntingtin generally adopts a random coil structure. It may also display regular structures such as α-helix or β-sheet. Oligomers may be made up of an α-helix core formed by the first seventeen amino-acids of huntingtin, with the polyQ being exposed and unstructured. Alternatively they may consist of small aggregates with buried polyQ, presumably with an antiparallel β-sheet rich structure. Microscopic aggregates (inclusions), composed of expanded huntingtin and other sequestered proteins, include amorphous aggregates and polymorphic amyloid conglomerates rich in β-sheets with antiparallel and/or parallel arrangements. Monomers, oligomers, and microscopic aggregates of expanded huntingtin, therefore, each comprise heterogeneous populations in terms of structure. They are additionally heterogeneous in size and morphology (see text).