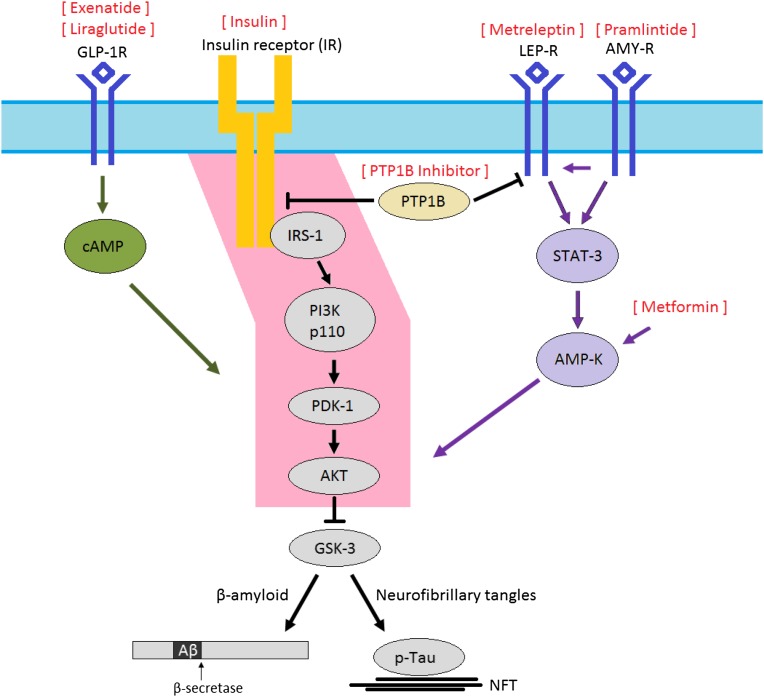
Figure 3.
All diabetes drugs are likely to have indirect effects in the CNS by affecting circulating concentrations of glucose and insulin. However, the especially intimate relationship between brain insulin resistance and AD, and the relative “paralysis” of brain insulin signaling through the IRS-1 → AKT pathway (pink) in MCI and AD, suggests that restoring signaling through this pathway with therapeutic agents originally developed for the treatment of diabetes may be of particular benefit. One approach is to overcome brain insulin resistance with exogenous insulin, but a theoretical concern of this approach is that in the long-run the chronic hyperinsulinemic environment will actually perpetuate brain insulin resistance. Other therapies, including GLP-1 agonists (e.g., exenatide, liraglutide), metformin, leptin analogs (metreleptin), amylin analogs (pramlintide), and PTP1B inhibitors may circumvent insulin-signaling impairment and reestablish signaling through the IRS-1 → AKT pathway. Peroxisome proliferator–activated receptor-γ agonists, such as rosiglitazone and pioglitazone, which reduce blood glucose by increasing GLUT-4 translocation but have been unsuccessful in improving outcomes in well-powered studies of AD, are somewhat removed from this impaired insulin-signaling pathway. NFT, neurofibrillary tangles.