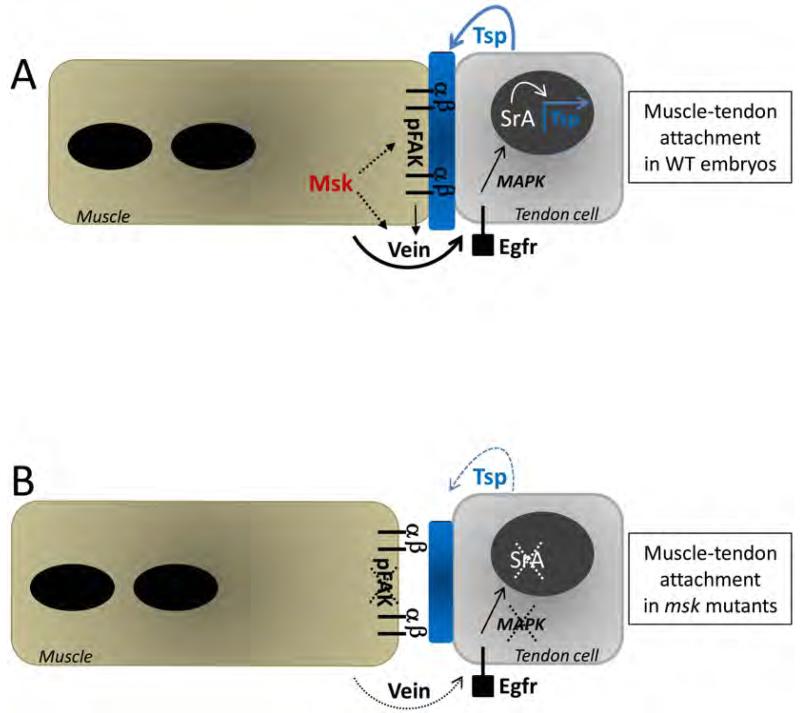
Figure 10. Model of Msk function at the muscle-tendon attachment site.
(A) In WT embryos, mature muscles associate with their corresponding tendon cells via an ECM-rich matrix (shown in blue) between the cells. Msk is required in the muscle and functions via secreted Vein to affect tendon cell function. Binding of Vein to the tendon-expressed Egfr activates a signaling cascade through activated MAPK. Activated MAPK translocates to the nucleus and with SrA, activates downstream genes to induce terminal differentiation in the tendon cells. Tight association of the muscle-expressed αPS2βPS integrin heterodimer to ECM proteins (such as the SrA target Tsp) is essential to withstand the force of muscle contraction. Msk may directly affect Vein secretion, accumulation and/or localization at the site of the muscle-tendon attachment site. Alternatively, Msk may affect Vein activity indirectly by modulating integrin-mediated adhesion. This adhesive function is required for Vein to properly activate the Egfr signaling pathway in the tendon cells. The effect of Msk on integrin-mediated adhesion may be direct or occur via phosphoproteins such as pFAK. Solid arrows denote previously published studies. Dotted arrows represent potential role(s) of Msk based upon our results. (B) In msk mutants, the ECM matrix (blue) is reduced in size and the muscle is no longer attached at the muscle-tendon attachment site. Terminal tendon cell differentiation does not occur as Sr and activated MAPK are dramatically reduced. Subsequently, Tsp expression and secretion is reduced, presumably resulting in smaller attachment sites. Within the muscle cells, pFAK is absent. Here, dotted lines and arrows denote a loss of protein and/or function due to a decrease in Msk protein.