Figure 2.
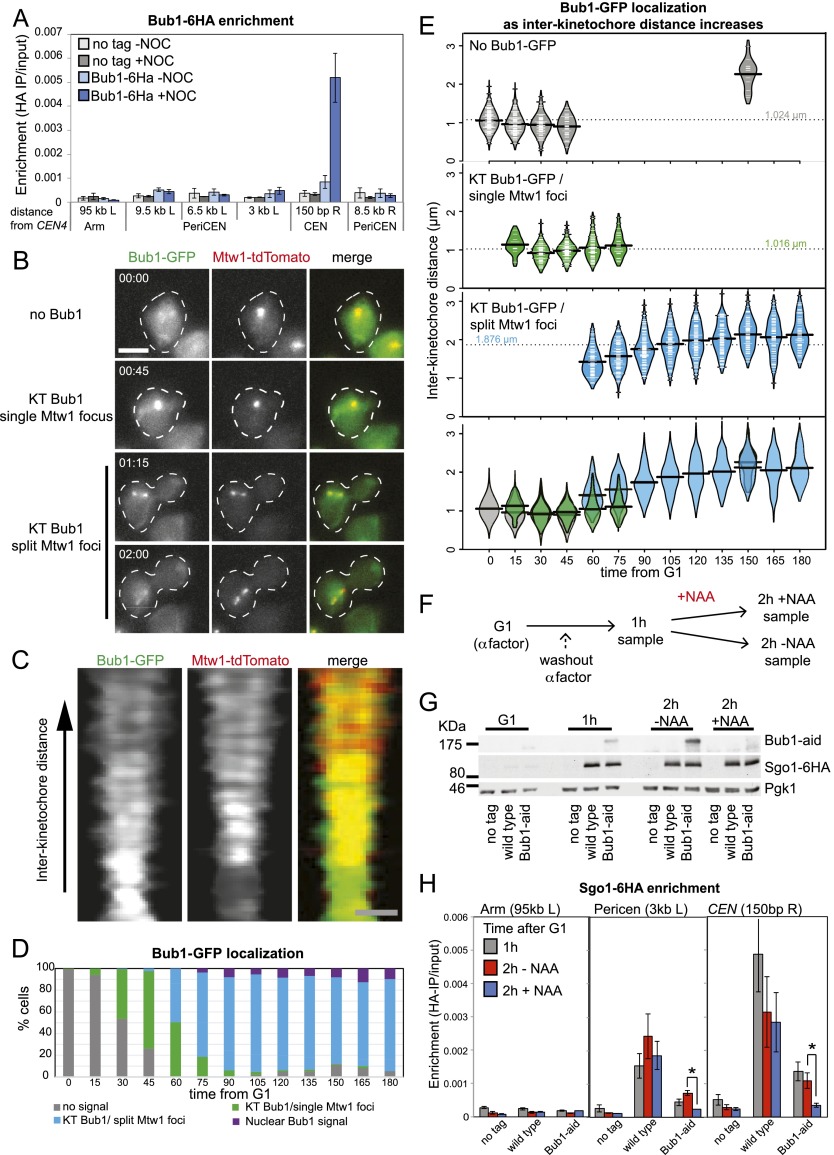
Bub1 is removed from kinetochores later than Sgo1 dissociates from the pericentromere. (A) Bub1 associates with centromeres in metaphase-arrested cells only in the absence of spindle tension. Cells (strain AM7449) carrying BUB1-6HA and pMET3-CDC20 and a no tag control (AM2508) were treated as described in Figure 1G. Bub1-6HA levels at the indicated sites were measured by ChIP-qPCR. The average of three experimental repeats is shown, with error bars representing standard error. (B–E) Bub1 is retained at kinetochores upon separation of kinetochore clusters. Cells carrying BUB1-yeGFP and MTW1-tdTomato (strain AM9229) were imaged on a microfluidics device at 15-min intervals after release from G1 arrest. (B) Cells exhibiting different types of Bub1-GFP localization at the indicated time points are shown. Bar, 5 μm. (C) Line scans across kinetochore foci of single cells were assembled from 100 images to generate a V plot showing Bub1-yeGFP localization as interkinetochore distance increases. Bar, 2 μm. (D) Bar chart with the fraction of cells with the indicated Bub1 localization at each time point is shown. (E) The distance between Mtw1-tdTomato signals and the localization of Bub1-yeGFP was scored in at least 90 cells for each time point. The bean plot shows the distribution of interkinetochore distances for which each localization type was scored. Lines within the beans represent individual cells. Beans for small sets of cells (N < 10) are not shown. The horizontal line represents the mean. (F–H) Continued Bub1 presence at kinetochores is required for Sgo1 localization at the pericentromere. (F) Scheme of the experiment is shown. Wild-type (AM6390) and bub1-aid OsTir1 (AM9096) cells carrying SGO1-6HA and a no tag control (AM2508), all carrying pMET3-CDC20, were released from G1 into methionine and nocodazole-containing medium. After 1 h, one-third of the culture was harvested for ChIP and Western blotting, the remaining culture was split, and NAA was added to one half. After 2 h total, the remaining cultures were harvested. (G) Western immunoblot analysis was performed with anti-aid, anti-HA, and anti-Pgk1 antibodies to confirm that Bub1 is degraded upon NAA treatment, but Sgo1 is not. Pgk1 is shown as a loading control. (H) ChIP-qPCR analysis of Sgo1 localization at the indicated sites on chromosome IV. The mean of three experimental repeats is shown, with error bars indicating standard error. Student’s t-test was used to calculate confidence values. (*) P < 0.05.