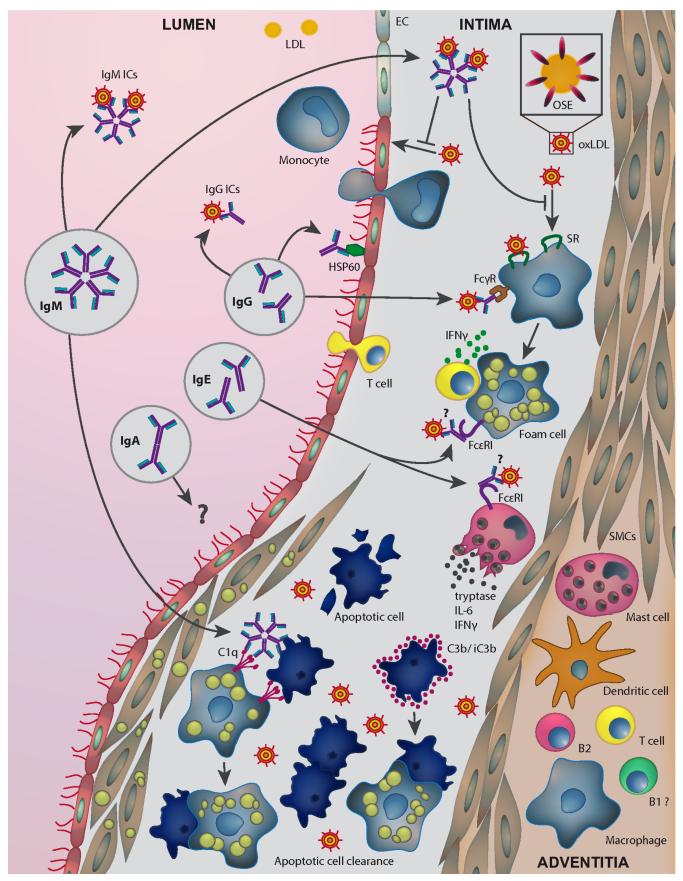
Figure 2. Role of immunoglobulins in lesion development.
Different immunoglobulins are present in atherosclerotic plaques, which are directed against relevant antigens such heat shock proteins (HSPs) and oxidized low-density lipoproteins (OxLDLs). IgM antibodies may mediate atheroprotection by neutralizing the proinflammatory properties of OxLDL, inhibiting the uptake of OxLDL by macrophages, and by promoting apoptotic cell clearance. Their protective capacity may be largely dependent on their ability to recognize oxidation-specific epitopes (OSEs) present on both OxLDL and apoptotic cells. OxLDL-specific IgG antibodies could activate macrophages via Fcγ receptor engagement, thereby promoting atherogenesis, but may also exhibit protective neutralizing capacities. IgM and IgG immune complexes with LDL carrying OSEs are also found in the circulation and may promote clearance of proatherogenic LDL particles. HSP60/65-specific IgG recognize stressed endothelial cells and induce damage via antibody-dependent cellular cytotoxicity. IgE antibodies activate mast cells and macrophages via the engagement FcεRI resulting in plaque destabilization. Specificities of IgE for atherosclerosis antigens, for example, OxLDL, remain to be shown. The role of IgA antibodies in atherosclerosis is still elusive. Opsonization of apoptotic cells with C3b and iC3b enhances their uptake by macrophages. EC indicates endothelial cells; IC, immune complexes; IFN-γ interferon-γ; IL-6, interleukin-6; SMCs, smooth muscle cells; and SR, scavenger receptors.