Abstract
Objective
Individuals with spinal cord injury (SCI) show structural and functional vascular maladaptations and muscle loss in their lower limbs. Angiogenic biomolecules play important roles in physiological and pathological angiogenesis, and are implicated in the maintenance of muscle mass. This study examined the responses of angiogenic molecules during upper-limb aerobic exercise in patients with SCI and in able-bodied (AB) individuals.
Methods
Eight SCI patients with thoracic lesions (T6–T12, ASIA A) and eight AB individuals performed an arm-cranking exercise for 30 minutes at 60% of their VO2max. Plasma concentrations of vascular endothelial growth factor (VEGF-A165), VEGF receptor 1 (sVEGFr-1), VEGF receptor 2 (sVEGFr-2), metalloproteinase 2 (MMP-2), and endostatin were measured at rest, after exercise, and at 1.5 and 3.0 hours during recovery.
Results
The two-way analysis of variance showed non-significant main effects of “group” and significant main effects of “time/exercise” for all angiogenic biomolecules examined (P < 0.01–0.001). The arm-cranking exercise significantly increased plasma concentrations of VEGF, sVEGFr-1, sVEGFr-2, MMP-2, and endostatin in both groups (P < 0.001–0.01). The magnitude of the increase was similar in both patients with SCI and AB individuals, as shown by the non-significant group × time interaction for all angiogenic parameters.
Conclusions
Upper-limb exercise (arm-cranking for 30 minutes at 60% of VO2max) is a sufficient stimulus to trigger a coordinated circulating angiogenic response in patients with SCI. The response of angiogenic molecules to upper-limb aerobic exercise in SCI appears relatively similar to that observed in AB individuals.
Keywords: Angiogenesis, Arm exercise, Exercise, Endostatin, Metalloproteinase, Spinal cord injury, Vascular endothelial growth factor
Introduction
Angiogenesis and arteriogenesis are regulated by the coordinated interaction between angiogenic and angiostatic molecules. Vascular endothelial growth factor (VEGF), metalloproteinases (MMPs), and endostatin are among the molecules involved in physiologic and pathologic angiogenic processes. More specifically, VEGF and its receptors (sVEGFr-1 and sVEGFr -2), and MMP-2, play important roles in microvascular remodeling,1–3 in regulating basal muscle capillarization, preserving vascular integrity, and maintaining and regenerating muscle mass.4 The expression of VEGF and MMPs is upregulated by catecholamines via activation of adrenergic receptor signaling pathways.1,5 Endostatin, on the other hand, acts as an inhibitor of the angiogenic process by directly interacting with the VEGF-2 receptor and preventing VEGF-induced endothelial cell migration and proliferation.6,7 It also restricts the formation of capillaries and suppresses the mobilization of endothelial progenitor cells (EPC) into the circulation.6
Individuals who have suffered a spinal cord injury (SCI) show impaired vascular control8 in the lower limbs and have an increased risk of developing cardiovascular disease. In addition, individuals with SCI show reduced capillarization and capillary diameter, and loss of muscle mass in the affected limbs.9 Exercise promotes angiogenesis and remodeling of the microvasculature and of the conduit vessels. Studies show that both VEGF and MMPs are important mediators of these processes in electrically stimulated skeletal muscles and during contraction-induced angiogenesis.10,11
A systemic rise in the concentrations of catecholamines, interleukins, and leukocytes may stimulate the expression of VEGF and MMPs.1,2,5,12,13 During exercise, individuals with SCI, compared with able-bodied individuals (AB), show autonomic nervous system dysfunction and alterations in the catecholamine and interleukin responses;14,15 thus, it is possible that the responses of angiogenic molecules to exercise might also be different in individuals with SCI. Previous studies examined the acute effects of “large muscle mass” exercise (i.e. cycling or running) on the levels of circulating angiogenic factors in both healthy and diseased populations.16–18 However, individuals with SCI cannot perform this type of exercise. The effects of a “small muscle” mass aerobic activity, such as an arm-cranking exercise, on angiogenic parameters have not been tested in these individuals, despite the fact that they regularly engage in this type of activity.
Therefore, the aim of this study was to examine the responses of angiogenic molecules in SCI and AB individuals during upper-limb (arm-cranking) aerobic exercise performed for 30 minutes at 60% of VO2max.
Methods
Participants
Eight individuals (seven male; one female) with spinal cord lesion between T6 and T12 (ASIA A; time since injury, 4.7 ± 1.3 years.) and eight AB controls (seven male; one female) participated in the study. The study conformed to the standards set out in the Declaration of Helsinki (2000) and was approved by The Institutional Ethical Committee. All participants provided written informed consent prior to the study and completed a health history questionnaire. All participants were healthy non-smokers who had normal arm function and no history of cardiovascular/pulmonary disease or any other chronic disorders. None were taking medications that might affect cardiovascular function.
Study design
The study was conducted over two separate days, scheduled 1 week apart. On the first day, the participants performed a maximal incremental test on an arm-ergometer to determine their maximal oxygen consumption (VO2max) and peak workload. On the second day, the participants performed an arm-cranking exercise for 30 minutes at 60% of their VO2max. Blood samples were collected before, immediately after, and at 1.5 and 3.0 hours after exercise. Plasma VEGF-A165 (referred to as VEGF from this point on), soluble VEGF receptor 1 (sVEGFr-1), soluble VEGF receptor 2 (sVEGFr-2), MMP-2, and endostatin levels were measured for all time points. The subjects were requested to abstain from caffeine and alcohol, as well as any physical activity for at least 24 hours before the tests.
Testing procedure and instrumentation
On the morning of day 1 (between 10 and 11 am), all participants reported to the exercise laboratory to assess their VO2max. The maximal incremental test was performed on an arm-ergometer (Monark Ergonomedic 881E, Varberg, Sweden) with the axis of the crank aligned with the height of the acromioclavicular joint and positioned at a distance that allowed the elbow to fully extend during cranking. Briefly, each participant started the test protocol at a workload of 25 W at 50 rpm. The workload was increased every 2 minutes for the first three stages (by 10 W for women and 15 W for men) and then every 1 minute. The test was terminated when the participant was no longer able to maintain the cranking rate. Respiratory gas exchange was measured breath-by-breath (Oxycon Pro, Jaeger, Germany) throughout the incremental test. The O2 and CO2 analyzers were calibrated prior to and after each test using ambient air and a gas of known composition (15.8 and 5.0%, respectively). Heart rate (HR) was continuously recorded using chest belt telemetry (Polar Electro, Kempele, Finland). The criteria for “maximum effort” were as follows: (i) a respiratory exchange ratio (RER) >1.05; (ii) visible exhaustion and the inability to sustain exercise despite continuous verbal encouragement; and (iii) a plateau in oxygen consumption (VO2) despite the increase in workload. All subjects attained at least two of the above criteria, thereby ensuring “maximum effort”.
On day 2, all participants reported to the laboratory at the same time (10:00 am) after an overnight fast. Following a 15-minute rest, a baseline blood sample was obtained from the antecubital vein and the participants performed the arm-cranking exercise at the workload corresponding to 60% of their individual maximal workload for 30 minutes. The patients maintained the cranking frequency throughout the maximal graded test and the sub-maximal arm-cranking exercise, by observing the rpm monitor fitted to the arm-ergometer. Blood samples were obtained immediately post-exercise, and at 1.5 and 3.0 hours of recovery.
Biochemical analyses
Blood samples (5 ml) were collected in EDTA tubes (VEGF, sVEGF-1, sVEGF-2, endostatin) or in heparin tubes (MMP-2). The samples were immediately centrifuged at 1800 × g at 4°C for 15 minutes (Centrifuge 5702R, Eppendorf AG, Hamburg, Germany). The plasma was removed, separated into aliquots, and stored at −80°C for later analysis of VEGF (pg/ml), sVEGFr-1 (pg/ml), sVEGFr-2 (pg/ml), endostatin (ng/ml), and MMP-2 (ng/ml). All biomolecules were analyzed in duplicate using Quantikine Human Immunoassay ELISA kits (R & D Systems, Minneapolis, MN, USA), according to the manufacturer's instructions. The intra-assay and the inter-assay coefficients of variation for all analyses were below 5.3 and 8.0%, respectively.
Statistical analysis
All data are presented as mean ± SD and were analyzed using Statistica version 7.0 (StatSoft Inc., Tulsa, OK, USA) software. Individuals with SCI and AB were compared in terms of their physical and physiological characteristics (age, height, body mass, body mass index, resting HR, and mean arterial pressure) and maximal graded exercise test variables (VO2peak, HRmax, total exercise time, and peak workload) using two-tailed independent t-tests. Two-way analysis of variance (ANOVA) with repeated measures (two groups × 4 time points) was used to examine the effects of “group” and “time” (exercise) on VEGF, sVEGFr-1, sVEGFr-2, MMP-2, and endostatin levels. The main effects were followed by Newman–Keuls post hoc tests (if needed) to locate significantly different means. The level of significance for all statistical analyses was set at P < 0.05. The effect sizes (ES) were calculated using partial eta squared (η2p). Small, medium, and large effects were reflected by η2p values >0.0099, 0.0588, and 0.1379, respectively.19,20
Results
Participants' characteristics
There were no significant differences between SCI and AB individuals in terms of age (34.3 ± 12.2 vs. 31.3 ± 7.8 years.), height (174 ± 8 vs. 177 ± 11 cm), body mass (74.0 ± 10.7 vs. 82.8 ± 16.0 kg), body mass index (24.6 ± 3.9 vs. 26.2 ± 3.1), resting HR (67.9 ± 6.9 vs. 65.5 ± 4.7 b/minute), and mean arterial pressure (87.5 ± 11.7 vs. 90.0 ± 7.8 mmHg). The performance data from the maximal arm-cranking test showed that VO2peak was lower in individuals with SCI than in AB individuals (18.2 ± 2.6 vs. 22.8 ± 5.3 ml/kg/minute; P < 0.05), whereas HRmax (170 ± 11 vs. 174 ± 14 b/minute), total exercise time (8.3 ± 1.2 vs. 9.9 ± 1.9 minutes), and peak workload (87 ± 17 vs. 103 ± 23 W) were not significantly different between the groups.
VEGF and VEGF receptors
Circulating plasma levels of VEGF (pg/ml), sVEGFr-1 (pg/ml), and sVEGFr-2 (pg/ml) in individuals with SCI and AB in response to the arm-cranking exercise are shown in Fig. 1. Two-way ANOVA indicated a non-significant main effect of “group” for VEGF (P = 0.412; η2p = 0.049 (small ES)), sVEGFr-1 (P = 0.703; η2p = 0.011 (small ES)), and sVEGFr-2 (P = 0.548; η2p = 0.026 (small ES)); however, the main effect of “time” was significant for all three variables (VEGF: P < 0.001, η2p = 0.392 (large ES); sVEGFr-1: P < 0.001, η2p = 0.427 (large ES); and sVEGFr-2: P < 0.01, η2p = 0.304 (large ES)). The “group × time” interaction was not significant for any of the variables (VEGF: P = 0.432; sVEGFr-1: P = 0.917; and sVEGFr-2: P = 0.372), suggesting that the effect of “time” (exercise) on the level of these angiogenic molecules was independent of the group examined.
Figure 1 .
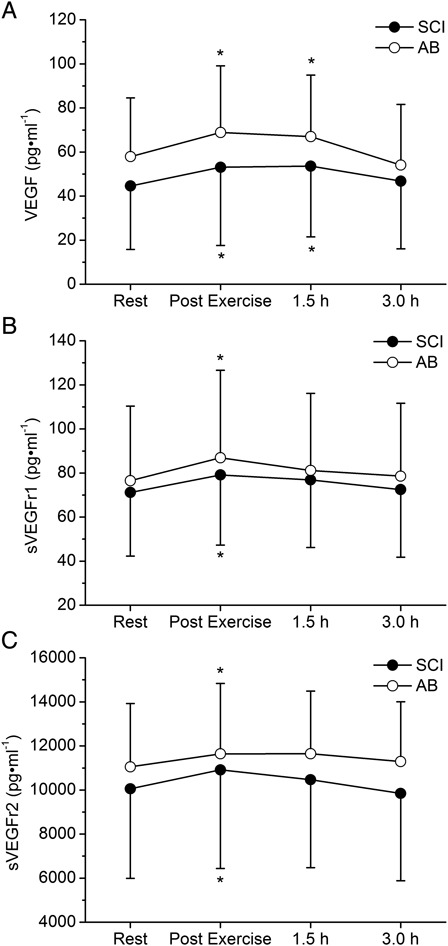
Values are presented as mean ± SD. Plasma concentrations (pg/ml) for VEGF (A), sVEGFr-1 (B), sVEGFr-2 (C) at rest, immediately after exercise, and at 1.5 and 3.0 hours after arm-exercise. Non-significant effect of “group” and “group × time” interaction; significant effect of “time” (P < 0.01); *P < 0.05 vs. resting values.
Post hoc analyses within the main effect of “time” revealed that the levels of VEGF (P < 0.01), sVEGFr-1 (P < 0.001), and sVEGFr-2 (P < 0.01) were significantly higher at the end of the arm-exercise compared to resting levels. VEGF levels remained significantly elevated at 1.5 hours post-exercise (P < 0.05 vs. rest), whereas sVEGFr-1 and sVEGFr-2 concentrations returned to resting levels.
Endostatin and MMP-2
The response of circulating plasma levels of endostatin (ng/ml) and MMP-2 (ng/ml) to the arm-cranking exercise are shown in Fig. 2. Two-way ANOVA identified non-significant main effects of “group” for endostatin (P = 0.774; η2p = 0.006 (trivial ES)) and MMP-2 (P = 0.772; η2p = 0.006 (trivial ES)) and significant main effects of “time” (endostatin: P < 0.001 and η2p = 0.585 (large ES); MMP-2: P < 0.001 and η2p = 0.386 (large ES)). The “group × time” interactions were not significant for endostatin (P = 0.256) or MMP-2 (P = 0.879). Post hoc analysis within the main effect of “time” showed that the circulating levels of endostatin and MMP-2 were higher (P < 0.001) at the “end of exercise” time point than at rest. Endostatin levels remained elevated at 1.5 and 3.0 hours post-exercise vs. rest (P < 0.001); whereas MMP-2 concentrations were elevated only at 1.5 hours post-exercise (P < 0.01) compared to rest.
Figure 2 .
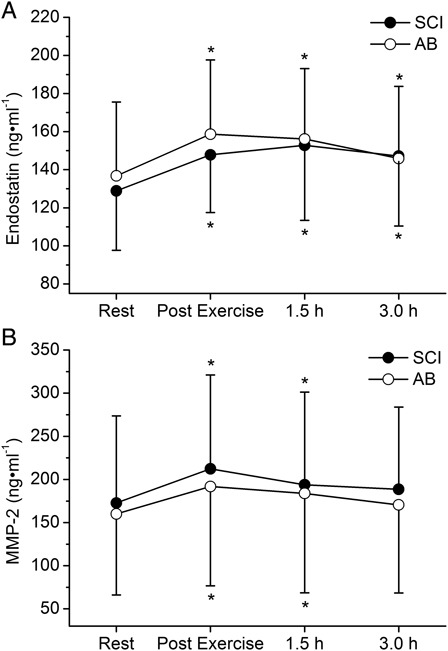
Values are presented as mean ± SD. Plasma concentrations (ng/ml) for endostatin (A) and MMP-2 (B) at rest, immediately after exercise, and at 1.5 and 3.0 hours after arm-exercise. Non-significant effect of “group” and “group × time” interaction; significant effect of “time” (P < 0.001); *P < 0.05 vs. resting values.
Discussion
This study examined whether an upper-limb (arm-cranking) aerobic exercise may provide a sufficient stimulus to increase the circulating levels of angiogenic biomolecules in individuals with SCI and AB. To the best of our knowledge, no study has examined the circulating levels of angiogenic factors in humans with SCI. The results of this study support the idea that exercise is a stimulus for angiogenesis in both individuals with SCI and AB. More specifically, we showed the following: (i) a “small muscle mass” (upper-limb) aerobic exercise session induces a moderate and transient increase in the levels of circulating VEGF and its receptors and stimulates the MMP system in both individuals with SCI and AB; and (ii) the magnitude of the changes in the levels of angiogenic factors in response to upper-limb aerobic exercise appears to be similar in SCI and AB individuals (as suggested by the non-significant group × time interaction).
Angiogenic biomolecules are essential components of exercise-induced angiogenesis in AB individuals. This study showed that the circulating levels of angiogenic factors in response to exercise also increased significantly in individuals with SCI; this implies that these factors are involved in exercise-induced vascular remodeling and angiogenesis in these individuals as well.21–25 This study is the first to investigate the responses of angiogenic molecules to exercise in individuals with SCI; thus, comparisons with previous studies using this population are not possible. However, earlier work in humans with intact spinal cords supports our findings in AB. These studies consistently report increases in VEGF, KDR and Flt-1, and MMP mRNA and/or protein in muscle tissue after a single bout of exercise.11,26,27 On the other hand, the results of studies looking at the effect of exercise on circulating levels of VEGF and its receptors are controversial, reporting either increases,16,18,28–32 decreases,18,30,33 or no change.17,34 Similarly, the results of previous studies looking at the acute effects of exercise on circulating MMPs report either stimulation of the MMP system or no effect.17,35–37 Previous studies are more consistent in terms of endostatin, demonstrating significant increases following either aerobic or resistance exercise.17,33,36 The reasons for these different results are unclear but may be attributed to the different exercise regimens, the timing of sample collection, or the site of sample collection in relation to the exercised limb. The main difference between the current and previous studies is that we used an arm-exercise and sampled from the vein, draining the primary muscle being exercised. The increase in plasma angiogenic factors after acute exercise observed in this study are consistent with the view that angiogenic compounds leave the muscle interstitium and enter the circulation.26,27,31,38
The most important finding of this study is that an upper-limb aerobic exercise session (at moderate intensity for 30 minutes) can trigger a coordinated angiogenic response of the VEGF system and increase the levels of circulating MMPs and endostatin in SCI. Stimulation of the VEGF system is of particular interest for patients with impaired peripheral vascular function. The rise in circulating VEGF levels may increase the number of circulating EPC7 by mobilizing EPC from the bone marrow. This increase in the amount of circulating EPC may in turn promote vasculogenesis within ischemic organs and improve regional blood flow, increase capillary density, and reduce muscle loss in the ischemic limbs.39 Furthermore, the induction of endostatin following arm-cranking exercise underscores the beneficial effects of this type of activity for individuals with SCI in preventing atherosclerosis40 and in regulating vascular tone.
There is evidence that exercise-induced increases in angiogenic factors are not limited to the muscle being exercised; rather, it is a systemic response. Animal and human studies have documented a rise in both circulating and tissue VEGF and MMP-2 levels in both exercised and non-exercised limbs.28,32,41 Furthermore, changes in the microvasculature of the untrained limb, such as an increase in capillary density and growth and an improved collateral circulation32,41,42 have been reported after training. In fact, Shen et al.41 reported that exercising a normal muscle may facilitate angiogenesis in untrained muscles with pathological ischemia. A possible explanation of the phenomenon of “remote angiogenesis” is that microvascular adaptations may result from the systemic rise in shear stress or the levels of circulating humoral substances, which in turn may elicit angiogenic response and/or vascular changes in the contralateral non-exercised muscles and/or un-perfused tissues.23,32,41–44 In support of these findings, Shen et al.41 reported that increased angiogenesis and capillarization in the non-exercising muscles is correlated with the upregulation of VEGF, while Ji et al.43 proposed that VEGF release may favor angiogenic growth in unperfused tissues. The above, along with our own findings, may be of importance for individuals with SCI, who demonstrate vascular maladaptations in their lower limbs and routinely perform arm-cracking exercise as part of their exercise program.
The origins and mechanisms underlying the increase in circulating angiogenic factors after arm-cranking exercise may only be speculated upon. Hypoxia,45 mechanical forces and muscle stretching,38,46 blood flow and shear stress,38,46 and increased sympathetic stimulation1,5 may all be involved in upregulating the production of angiogenic factors. Furthermore, several cytokines (such as interleukins and growth hormone)2,47 and a systemic rise in leukocytes12,13 may also contribute to the increased plasma levels of VEGF, and its receptors, MMP-2, and endostatin. On the basis of previous work, these multiple signals are generated during arm-exercise in individuals with SCI with thoracic lesions14,15,48–51; thus, the increase in plasma angiogenic molecules in individuals with SCI observed in this study is well supported. Comparative studies between SCI and AB individuals show that some signals are exaggerated during exercise in individuals with SCI (i.e. catecholamines, sympathetic activity, and peak anterograde shear rate),14,48 whereas others are depressed (i.e. mean shear rate)48 and some remain unchanged (i.e. interleukins-6 and blood flow to the exercising muscles).49,50
This study has some potential limitations. First, our findings are limited to individuals with complete thoracic lesions. It is possible that individuals with SCI with higher-level (above T6) or incomplete lesions may show different exercise responses of angiogenic biomolecules due to blunted sympathetic nervous system (SNS) activity (tetraplegics)14 or a lesser degree of autonomic dysfunction (incomplete injuries). Second, a fairly small sample size was used and a relatively large variance in the data was observed. However, the variance is within the low-to-mid range of that previously reported for the angiogenic parameters examined in this study.52–55 Third, we measured circulating angiogenic parameters. The assessment of these molecules in the tissues would provide additional insight into the effects of arm exercise on angiogenesis in the upper and lower limbs. Finally, although our study provides evidence that arm-exercise triggers a circulating angiogenic response in individuals with SCI, whether this will cause vascular adaptations in the legs of SCI remains to be tested.
In conclusion, an upper-limb exercise session comprising of moderate arm-cranking for 30 minutes is sufficient to increase the circulating levels of angiogenic growth factors in individuals with SCI. The response, however, of these angiogenic molecules to upper-limb aerobic exercise in SCI appears relatively similar to that in AB (as suggested by the lack of group × time interaction). Yet, trivial/small (non-significant) effect sizes were observed when we compared the concentrations of angiogenic molecules between the two groups. The findings of this study are of importance to individuals with SCI, and to those who restrict their activity to upper-limb exercise or have impaired lower limb function. Additional research (i.e. training interventions) is needed to examine whether the rise in the levels of circulating angiogenic biomolecules after arm exercise in individuals with SCI may promote favorable microvascular adaptations in their lower paralyzed limbs.
Acknowledgments
The authors thank all the participants for their cooperation and their voluntary participation in this study. This study was partially supported by the Research Committee of the Aristotle University of Thessaloniki, scholarships and sponsorships of research programs 2011 (Excellence Awards 2011; code number 50141).
References
- 1.Hattori Y, Yamamoto S, Matsuda N. Sympathetic control of VEGF angiogenic signaling: dual regulations by alpha 2-adrenoceptor activation? Circ Res 2007;101(7):642–4 [DOI] [PubMed] [Google Scholar]
- 2.Ferrara N, Davis-Smyth T. The biology of vascular endothelial growth factor. Endocr Rev 1997;18(1):4–25 [DOI] [PubMed] [Google Scholar]
- 3.Stetler-Stevenson WG Matrix metalloproteinases in angiogenesis: a moving target for therapeutic intervention. J Clin Invest 1999;103(9):1237–41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tang K, Breen EC, Gerber HP, Ferrara NM, Wagner PD. Capillary regression in vascular endothelial growth factor-deficient skeletal muscle. Physiol Genom 2004;18(1):63–9 [DOI] [PubMed] [Google Scholar]
- 5.Yang EV, Sood AK, Chen M, Li Y, Eubank TD, Marsh CB, et al. Norepinephrine up-regulates the expression of vascular endothelial growth factor, matrix metalloproteinase (MMP)-2, and MMP-9 in nasopharyngeal carcinoma tumor cells. Cancer Res 2006;66(21):10357–64 [DOI] [PubMed] [Google Scholar]
- 6.Seppinen L, Pihlajaniemi T. The multiple functions of collagen XVIII in development and disease. Matrix Biol 2011;30(2):83–92 [DOI] [PubMed] [Google Scholar]
- 7.Kim YM, Hwang S, Pyun BJ, Kim TY, Lee ST, Gho YS, et al. Endostatin blocks vascular endothelial growth factor-mediated signaling via direct interaction with KDR/Flk-1. J Biol Chem 2002;277(31):27872–9 [DOI] [PubMed] [Google Scholar]
- 8.Olive JL, McCully KK, Dudley GA. Blood flow response in individuals with incomplete spinal cord injuries. Spinal Cord 2002;40(12):639–45 [DOI] [PubMed] [Google Scholar]
- 9.Ruschkewitz Y, Gefen A. Cell-level temperature distributions in skeletal muscle post spinal cord injury as related to deep tissue injury. Med Biol Eng Comput 2010;48(2):113–22 [DOI] [PubMed] [Google Scholar]
- 10.Amaral SL, Linderman JR, Morse MM, Greene AS. Angiogenesis induced by electrical stimulation is mediated by angiotensin II and VEGF. Microcirculation 2001;8(1):57–67 [PubMed] [Google Scholar]
- 11.Haas TL, Milkiewicz M, Davis SJ, Zhou AL, Egginton S, Brown MD, et al. Matrix metalloproteinase activity is required for activity-induced angiogenesis in rat skeletal muscle. Am J Physiol Heart Circ Physiol 2000;279(4):H1540–7 [DOI] [PubMed] [Google Scholar]
- 12.Reeps C, Pelisek J, Seidl S, Schuster T, Zimmermann A, Kuehnl A, et al. Inflammatory infiltrates and neovessels are relevant sources of MMPs in abdominal aortic aneurysm wall. Pathobiology 2009;76(5):243–52 [DOI] [PubMed] [Google Scholar]
- 13.Iacobaeus E, Amoudruz P, Strom M, Khademi M, Brundin L, Hillert J, et al. The expression of VEGF-A is down regulated in peripheral blood mononuclear cells of patients with secondary progressive multiple sclerosis. PLoS One 2011;6(5):e19138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schmid A, Huonker M, Barturen JM, Stahl F, Schmidt-Trucksass A, Konig D, et al. Catecholamines, heart rate, and oxygen uptake during exercise in persons with spinal cord injury. J Appl Physiol 1998;85(2):635–41 [DOI] [PubMed] [Google Scholar]
- 15.Kouda K, Furusawa K, Sugiyama H, Sumiya T, Ito T, Tajima F, et al. Does 20-minute arm crank ergometer exercise increase plasma interleukin-6 in individuals with cervical spinal cord injury? Eur J Appl Physiol 2012;112(2):597–604 [DOI] [PubMed] [Google Scholar]
- 16.Kraus RM, Stallings HW, Yeager RC, Gavin TP. Circulating plasma VEGF response to exercise in sedentary and endurance-trained men. J Appl Physiol 2004;96(4):1445–50 [DOI] [PubMed] [Google Scholar]
- 17.Suhr F, Brixius K, de Marees M, Bolck B, Kleinoder H, Achtzehn S, et al. Effects of short-term vibration and hypoxia during high-intensity cycling exercise on circulating levels of angiogenic regulators in humans. J Appl Physiol 2007;103(2):474–83 [DOI] [PubMed] [Google Scholar]
- 18.Weissgerber TL, Davies GA, Roberts JM. Modification of angiogenic factors by regular and acute exercise during pregnancy. J Appl Physiol 2010;108(5):1217–23 [DOI] [PubMed] [Google Scholar]
- 19.Richardson JT Eta squared and partial eta squared as measures of effect size in educational research. Educ Res Rev 2011;6(2):135–47 [Google Scholar]
- 20.Cohen J Statistical power analysis for the behavioural sciences. 2nd ed Hillsdale, NJ: Lawrence Erlbaum Associates; 1988 [Google Scholar]
- 21.Martin TP, Stein RB, Hoeppner PH, Reid DC. Influence of electrical stimulation on the morphological and metabolic properties of paralyzed muscle. J Appl Physiol 1992;72(4):1401–6 [DOI] [PubMed] [Google Scholar]
- 22.Duffell LD, Rowlerson AM, de Donaldson N, Harridge SD, Newham DJ. Effects of endurance and strength-directed electrical stimulation training on the performance and histological properties of paralyzed human muscle: a pilot study. Muscle Nerve 2010;42(5):756–63 [DOI] [PubMed] [Google Scholar]
- 23.Rowley NJ, Dawson EA, Hopman MT, George K, Whyte GP, Thijssen DH, et al. Conduit diameter and wall remodelling in elite athletes and spinal cord injury. Med Sci Sports Exerc 2012;44(5):844–9 [DOI] [PubMed] [Google Scholar]
- 24.Jae SY, Heffernan KS, Lee M, Fernhall B. Arterial structure and function in physically active persons with spinal cord injury. J Rehabil Med 2008;40(7):535–8 [DOI] [PubMed] [Google Scholar]
- 25.Thijssen DH, Ellenkamp R, Smits P, Hopman MT. Rapid vascular adaptations to training and detraining in persons with spinal cord injury. Arch Phys Med Rehabil 2006;87(4):474–81 [DOI] [PubMed] [Google Scholar]
- 26.Gavin TP, Robinson CB, Yeager RC, England JA, Nifong LW, Hickner RC. Angiogenic growth factor response to acute systemic exercise in human skeletal muscle. J Appl Physiol 2004;96(1):19–24 [DOI] [PubMed] [Google Scholar]
- 27.Hiscock N, Fischer CP, Pilegaard H, Pedersen BK. Vascular endothelial growth factor mRNA expression and arteriovenous balance in response to prolonged, submaximal exercise in humans. Am J Physiol Heart Circ Physiol 2003;285(4):H1759–63 [DOI] [PubMed] [Google Scholar]
- 28.Nemet D, Hong S, Mills PJ, Ziegler MG, Hill M, Cooper DM. Systemic vs. local cytokine and leukocyte responses to unilateral wrist flexion exercise. J Appl Physiol 2002;93(2):546–54 [DOI] [PubMed] [Google Scholar]
- 29.Czarkowska-Paczek B, Bartlomiejczyk I, Przybylski J. The serum levels of growth factors: PDGF, TGF-beta and VEGF are increased after strenuous physical exercise. J Physiol Pharmacol 2006;57(2):189–97 [PubMed] [Google Scholar]
- 30.Bailey AP, Shparago M, Gu JW. Exercise increases soluble vascular endothelial growth factor receptor-1 (sFlt-1) in circulation of healthy volunteers. Med Sci Monit 2006;12(2):CR45–50 [PubMed] [Google Scholar]
- 31.Gavin TP, Drew JL, Kubik CJ, Pofahl WE, Hickner RC. Acute resistance exercise increases skeletal muscle angiogenic growth factor expression. Acta Physiol (Oxf) 2007;191(2):139–46 [DOI] [PubMed] [Google Scholar]
- 32.Hoier B, Rufener N, Bojsen-Moller J, Bangsbo J, Hellsten Y. The effect of passive movement training on angiogenic factors and capillary growth in human skeletal muscle. J Physiol 2010;588(Pt 19):3833–45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gu JW, Fortepiani LA, Reckelhoff JF, Adair TH, Wang J, Hall JE. Increased expression of vascular endothelial growth factor and capillary density in hearts of spontaneously hypertensive rats. Microcirculation 2004;11(8):689–97 [DOI] [PubMed] [Google Scholar]
- 34.Wood RE, Sanderson BE, Askew CD, Walker PJ, Green S, Stewart IB. Effect of training on the response of plasma vascular endothelial growth factor to exercise in patients with peripheral arterial disease. Clin Sci (Lond) 2006;111(6):401–9 [DOI] [PubMed] [Google Scholar]
- 35.Koskinen SO, Hoyhtya M, Turpeenniemi-Hujanen T, Martikkala V, Makinen TT, Oksa J, et al. Serum concentrations of collagen degrading enzymes and their inhibitors after downhill running. Scand J Med Sci Sports 2001;11(1):9–15 [DOI] [PubMed] [Google Scholar]
- 36.Suhr F, Rosenwick C, Vassiliadis A, Bloch W, Brixius K. Regulation of extracellular matrix compounds involved in angiogenic processes in short- and long-track elite runners. Scand J Med Sci Sports 2010;20(3):441–8 [DOI] [PubMed] [Google Scholar]
- 37.Nourshahi M, Hedayati M, Ranjbar K. The correlation between resting serum leptin and serum angiogenic indices at rest and after submaximal exercise. Regul Pept 2012;173(1–3):6–12 [DOI] [PubMed] [Google Scholar]
- 38.Hellsten Y, Rufener N, Nielsen JJ, Hoier B, Krustrup P, Bangsbo J. Passive leg movement enhances interstitial VEGF protein, endothelial cell proliferation, and eNOS mRNA content in human skeletal muscle. Am J Physiol Regul Integr Comp Physiol 2008;294(3):R975–82 [DOI] [PubMed] [Google Scholar]
- 39.Krassioukov AV, Karlsson AK, Wecht JM, Wuermser LA, Mathias CJ, Marino RJ. Assessment of autonomic dysfunction following spinal cord injury: rationale for additions to International Standards for Neurological Assessment. J Rehabil Res Dev 2007;44(1):103–12 [DOI] [PubMed] [Google Scholar]
- 40.Alexander MS, Biering-Sorensen F, Bodner D, Brackett NL, Cardenas D, Charlifue S, et al. International standards to document remaining autonomic function after spinal cord injury. Spinal Cord 2009;47(1):36–43 [DOI] [PubMed] [Google Scholar]
- 41.Shen M, Gao J, Li J, Su J. Effect of ischaemic exercise training of a normal limb on angiogenesis of a pathological ischaemic limb in rabbits. Clin Sci (Lond) 2009;117(5):201–8 [DOI] [PubMed] [Google Scholar]
- 42.Hudlicka O, Graciotti L, Fulgenzi G, Brown MD, Egginton S, Milkiewicz M, et al. The effect of chronic skeletal muscle stimulation on capillary growth in the rat: are sensory nerve fibres involved? J Physiol 2003;546(Pt 3):813–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ji JW, Mac Gabhann F, Popel AS. Skeletal muscle VEGF gradients in peripheral arterial disease: simulations of rest and exercise. Am J Physiol Heart Circ Physiol 2007;293(6):21. [DOI] [PubMed] [Google Scholar]
- 44.Padilla J, Simmons GH, Bender SB, Arce-Esquivel AA, Whyte JJ, Laughlin MH. Vascular effects of exercise: endothelial adaptations beyond active muscle beds. Physiology (Bethesda) 2011;26(3):132–45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dor Y, Porat R, Keshet E. Vascular endothelial growth factor and vascular adjustments to perturbations in oxygen homeostasis. Am J Physiol Cell Physiol 2001;280(6):C1367–74 [DOI] [PubMed] [Google Scholar]
- 46.Brown MD, Hudlicka O. Modulation of physiological angiogenesis in skeletal muscle by mechanical forces: involvement of VEGF and metalloproteinases. Angiogenesis 2003;6(1):1–14 [DOI] [PubMed] [Google Scholar]
- 47.Li A, Dubey S, Varney ML, Dave BJ, Singh RK. IL-8 directly enhanced endothelial cell survival, proliferation, and matrix metalloproteinases production and regulated angiogenesis. J Immunol 2003;170(6):3369–76 [DOI] [PubMed] [Google Scholar]
- 48.Thijssen DH, Green DJ, Steendijk S, Hopman MT. Sympathetic vasomotor control does not explain the change in femoral artery shear rate pattern during arm-crank exercise. Am J Physiol Heart Circ Physiol 2009;296(1):H180–5 [DOI] [PubMed] [Google Scholar]
- 49.Zafeiridis A, Vasiliadis AV, Doumas A, Galanis N, Christoforidis T, Kyparos A, et al. Muscle perfusion of posterior trunk and lower-limb muscles at rest and during upper-limb exercise in spinal cord-injured and able-bodied individuals. Spinal Cord 2012;50(11):822–6 [DOI] [PubMed] [Google Scholar]
- 50.Umemoto Y, Furusawa K, Kouda K, Sasaki Y, Kanno N, Kojima D, et al. Plasma IL-6 levels during arm exercise in persons with spinal cord injury. Spinal Cord 2011;49(12):1182–7 [DOI] [PubMed] [Google Scholar]
- 51.Banno M, Nakamura T, Furusawa K, Ogawa T, Sasaki Y, Kouda K, et al. Wheelchair half-marathon race increases natural killer cell activity in persons with cervical spinal cord injury. Spinal Cord 2012;50(7):533–7 [DOI] [PubMed] [Google Scholar]
- 52.Felmeden DC, Spencer CG, Belgore FM, Blann AD, Beevers DG, Lip GY. Endothelial damage and angiogenesis in hypertensive patients: relationship to cardiovascular risk factors and risk factor management. Am J Hypertens 2003;16(1):11–20 [DOI] [PubMed] [Google Scholar]
- 53.Kim SY, Lee SH, Park S, Kang SM, Chung N, Shim WH, et al. Vascular endothelial growth factor, soluble FMS-like tyrosine kinase 1, and the severity of coronary artery disease. Angiology 2011;62(2):176–83 [DOI] [PubMed] [Google Scholar]
- 54.Wada H, Satoh N, Kitaoka S, Ono K, Morimoto T, Kawamura T, et al. Soluble VEGF receptor-2 is increased in sera of subjects with metabolic syndrome in association with insulin resistance. Atherosclerosis 2010;208(2):512–7 [DOI] [PubMed] [Google Scholar]
- 55.Derosa G, D'Angelo A, Scalise F, Avanzini MA, Tinelli C, Peros E, et al. Comparison between metalloproteinases-2 and -9 in healthy subjects, diabetics, and subjects with acute coronary syndrome. Heart Vessels 2007;22(6):361–70 [DOI] [PubMed] [Google Scholar]


