Abstract
We quantified the magnitude and investigated mechanisms regulating intrinsic force (IF) in human airway smooth muscle (hASM). IF was identified by reducing extracellular calcium (Ca2+) concentration to nominally zero in freshly isolated isometrically mounted 2 mm human bronchi. Our results show: (1) the magnitude of IF is ~ 50% of the maximal total force elicited by acetylcholine (10-5 M) and is epithelial-independent, (2) IF can also be revealed by β-adrenergic activation (isoproterenol), non-specific cationic channel blockade (La3+) or L-type voltage gated Ca2+ channel blockade (nifedipine), (3) atropine, indomethacin, AA– 861, or pyrilamine did not affect IF, (4) IF was reduced by the intracellular Ca2+ ([Ca2+]i) chelating agent BAPTA-AM, (5) ω-conotoxin had no effect on IF. In studies in cultured hASM cells nominally zero Ca2+ buffer and BAPTA-AM reduced [Ca2+]i but isoproterenol and nifedipine did not. Taken together these results indicate that rapid reduction of [Ca2+]i reveals a permissive relationship between extracellular Ca2+, [Ca2+]i and IF. However IF can be dissipated by mechanisms effecting Ca2+ sensitivity. We speculate that an increase of IF, a fundamental property of ASM, could be related to human airway clinical hyperresponsiveness and must be accounted for in in vitro studies of hASM.
Keywords: Intrinsic force, human, airway smooth muscle, asthma, intracellular calcium concentration [Ca2+]i
1. Introduction
Though in health the in vivo human airway smooth muscle (ASM) contractile apparatus is considered to be minimally active several lines of evidence suggest otherwise. Of course, in airway diseases such as COPD and asthma enhanced airway luminal narrowing is caused by excessive shortening of ASM. The excessive shortening could result from exposure to spasmogens (Driver et al., 1993; Lam et al., 1988; Mattoli et al., 1991; Murray et al., 1986; Wenzel et al., 1991; White and Eiser, 1983), increased ASM mass (Hogg et al., 2004), increased myocyte contractility (Opazo Saez et al., 2000), reduced load on ASM (Bosse et al., 2007), or length adaptation of myocytes to passively or actively shortened lengths (McParland et al., 2005). However, another property of ASM which could affect nascent airway diameter is the force generation in the absence of concurrent neural or humeral (i.e. spasmogen) stimulation. This latter property has been referred to as resting tonus or IF. The vast majority of human ASM studies on in vitro force generation and relaxation ignore the presence of IF and reference the resting force (forceresting) to be zero in unstimulated ASM strips or bronchial rings set at an optimal resting length or load to maximize active force.
In 1937, an in vitro study on thin slices of human lung noted adrenaline dilated (sic unstimulated) bronchi (Sollmann and Gilbert, 1937). Later, Hawkins and Schild described spontaneous “tonus” of cut rings of 2nd to 5th generation bronchi as sympathomimetic compounds and aminophylline decreased an applied resting tension (Hawkins and Schild, 1951). More recently high-resolution computed tomography (Scichilone et al., 2001) has reaffirmed the in vivo presence of “resting” bronchomotor tone initially described by Fish el al (Fish et al., 1981) and later Skloot et al (Skloot et al., 1995). These imaging studies as well as human pulmonary function studies, note that deep inspiration reduces an active bronchomotor force in normal humans, a process that is less effective in asthmatic subjects. Though this finding could be due in some part to the elastic nature of airway tissue, sufficient in vitro studies (Rabe et al., 1995) support the concept that human airway smooth muscle is at least “constitutively” contracted (i.e. active cross bridge cycling) in health. As the in vivo caliber of small human airways is considered to result from the balance between radial traction forces due to interdependent tethering between the airway wall and the surrounding parenchyma and both airway elastance and smooth muscle contractile forces increases in IF due to inflammatory mediators or cytoarchitectural remodeling could led to increased airway resistance.
In this study we sought to determine the magnitude of in vitro IF generation that is not due to elasticity as well as to clarify the role of extracellular Ca2+, prostaglandin and leukotriene synthesis on airway myocyte IF. As total force generation by ASM effects airway caliber it is important that investigations on the role of specific bronchoconstrictor substances strictly delineate the magnitude and cause of IF prior to drawing conclusions about the specific mechanism of the bronchoconstrictor substance at hand. To that extent we systematically examined the role of cysteinyl–leukotrienes, histamine, cyclooxygenase products, extracellular Ca2+, and dihydropyridine Ca2+ channel antagonist on the existence of IF in 2 mm human airway.
2. Methods and materials
2.1. Human bronchi isolation
Human bronchi were obtained from surgical specimens in accordance with procedures approved by the Mayo Clinic Foundation Institutional Review Board. Tissues obtained were incidental to the patient's surgery and were discarded by the surgical pathologist. All tissues were immersed in ice-cold HBSS (Hanks balanced salt solution with 2.25 mM CaCl2, 0.8 mM MgSO4 and 12 mM glucose, pH 7.4). 3rd to 6th generation bronchi were freed from adherent tissue using a dissecting microscope. To eliminate interdependence of airway diameter due to lung volume we surgically dissected 5th generation epithelium-intact and epithelial-denuded human bronchi from discarded specimens. We removed epithelial cells by gently rubbing the rings with cotton swab moistened with HBSS. Successful removal of epithelium cells was confirmed histologically. Bronchial segments (4-5 mm length, 1-2 mm diameter) were prepared and kept in ice-cold oxygenated HBSS until use in experimental protocols.
2.2. Force measurements
Human bronchi were obtained from surgical specimens as described above. Human bronchial rings were mounted vertically between two stainless steel wire supports, one fixed the other attached to an isometric force transducer (model FT03, Grass Instruments, Quincy, MA). The organ baths contained 10 ml physiological salt solution (PSS) of the following composition (in mM): 0.81 MgSO4, 1.19 KH2PO4, 3.36 KCl, 2.39 CaCl2, 106.9 NaCl, 25.45 NaHCO3 and 5.5 Dextrose. The baths were maintained at 37°C and bubbled continuously with 5% CO2 in O2, pH 7.4. Transducer outputs were amplified, recorded on a polygraph, digitized, and analyzed with a microcomputer.
2.3. Determination of optimal forceresting
To determine optimal forceresting in the absence of IF as previously reported (Wylam et al., 1993), the rings were placed in an organ bath containing PSS with 0.0 mM Ca2+ (0 CaPSS). The force was transiently adjusted to 88.2 mN (9.0 g) for 1 to 2 min and then reduced to 0.0 mN (0.0 g). The rings were then incubated for 1 h in 0 CaPSS at 2.45 mN (0.25 g) forceresting. Active force was recorded for 5 min after replacing the buffer with PSS containing 60 mM KCl. The rings were then rinsed three times in 0 CaPSS. After returning to base-line force, the ring was again stretched transiently to 88.2 mN (9.0 g) and then returned to 0.0 mN (0.0 g). The forceresting was set at a target value for 30 min; during this time, the force was readjusted every 10 min to its target value. Using this approach, forceresting of 4.9 mN (0.5 g), 9.8 mN (1.0 g), 19.6 mN (2.0 g), 29.4 mN (3.0 g) and 39.2 mN (4.0 g) were studied sequentially in each ring. Optimal forceresting was determined as the minimal forceresting necessary to give a maximal active force when compared by multivariate analysis, which was 4.9 mN (0.5 g). All subsequent studies were performed using the optimal forceresting of 4.9 mN (0.5 g).
2.4. Measurement of IF and active force in epithelium intact bronchi
Human bronchi were prepared and rings were mounted and bathed in 10 ml organ baths with PSS (Control) or PSS containing indomethacin, AA–861, pyrilamine or indomethacin and AA–861 as described above at an optimal forceresting of 4.9 mN (0.5 g). Tissues were equilibrated for at least 60 min to establish a stable baseline. During this time the rings were readjusted every 15 min to the target value of 4.9 mN (0.5 g). After the initial 60 min equilibration period, PSS was rapidly replaced by 0 CaPSS (Control) or 0 CaPSS containing indomethacin, AA–861, pyrilamine or indomethacin and AA–861 to determine the level of IF associated with each preparation. IF was determined to be decrease in forceresting. Tissues were then washed repeatedly in PSS or PSS containing the different drugs for an additional 30 min to again establish a stable baseline and at this time ACh was added at a final concentration of 10 μM to determine the active force for each ring.
2.5. Human bronchi response to altered extracellular Ca2+
Human bronchi rings were placed in PSS and the tension was set to 4.9 mN (0.5 g). After 1 h equilibration, the buffer was rapidly replaced by 0CaPSS for at least 30 min to equilibrate. The 0 CaPSS was then replaced with buffer containing 0.1, 0.25, 0.5, 1.0, 2.5, 5.0 or 10.0 mM Ca2+. The rings were bathed in each buffer for 20 min to attain a stable force level. The tension was recorded continuously for 4 h to assess the influence of each extracellular Ca2+ concentration.
2.6. Measurement of IF in epithelium intact bronchi
Human bronchi were prepared and rings were mounted and bathed in 10 ml organ baths with PSS described above at a forceresting of 4.9 mN (0.5 g). Tissues were equilibrated for at least 60 min to establish a stable baseline tension. During this time, the rings were readjusted every 15 min to their target value. After the initial 60 min equilibration period, PSS was rapidly replaced by 0 CaPSS or PSS containing the different drugs (lanthanum, BAPTA–AM, isoproterenol, atropine, nifedipine, indomethacin, AA–861, pyrilamine and ω-conotoxin) to determine the level of IF associated with each preparation.
2.7. Determination of smooth muscle cell [Ca2+]i in isolated human bronchi
Changes in global [Ca2+]i in smooth muscle of isolated human bronchi in response to Ca2+-channel modulators or to changes in extracellular [Ca2+] were determined. For these studies, individual bronchial strips were placed in the chamber of a mini-blood vessel superfusion apparatus (Living Systems, Burlington, VT) which had been modified to hold and position each strip securely. Human bronchial rings were prepared as described above and each ring was cut open to yield a strip. Each strip was tied at either end between two stainless steel wires supports spaced about 1 cm apart, given minimal stretch, and then immersed into the chamber. The chamber apparatus was then placed onto the stage of an inverted microscope, which employed a 20× S-Fluor lens. The strip was then superfused at 5 ml/min with Krebs-Ringer Solution aerated with 5% CO2 in oxygen and maintained at 25°C. After 30 min equilibration, superfusion was stopped and the strip was loaded with Fura 2-AM by placing 500 μl Krebs-Ringer Solution containing 5 μM Fura-2 AM with an equal volume of a 25% (w/v) solution of pluronic acid into the chamber and aerated with 5% CO2 in oxygen and maintained at 25°C, and put in the dark for 60 min. The balance of the experiment was carried out using continuous superfusion with aerated Krebs-Ringer solution at 37°C. After 45 min equilibration, the experimental protocols were initiated and [Ca2+]i levels were approximated using the ratios of the 510-nm emission from the strips alternately excited at 340 and 380 nm obtained at a sampling rate of 3 Hz using a photomultiplier system (IonOptix, Milton, MA). After each protocol, the experimental strip was removed and replaced with a second, non-Fura-2-loaded strip for determination of background fluorescence levels under experimental conditions. Background-corrected changes in global [Ca2+]i within each strip in response to experimental manipulation were approximated using this method.
2.8. Effect of nifedipine on KCL induced contraction in epithelium intact human bronchi
Human bronchi were prepared and rings were mounted and bathed in 10 ml organ baths with PSS as described above at a forceresting of 4.9 mN (0.5 g). After the initial equilibration period, adding KCl at a final concentration of 60 mM bronchial rings was contracted. When the response reached a plateau, the rings were washed by exchanging the medium until the basal tone was reestablished. The bronchial rings were then incubated for 30 min with PSS containing atropine 10-6 M, nifedipine was then added at different concentrations (0,10-9,10-8, 10-7, 10-6,10-5 M), again incubated for 30 min to establish a stable baseline. KCL was again added to a final concentration of 60 mM to determine the force developed for each ring. The second KCl induced contraction was compared to the first KCl induced contraction to determine the effects of nifedipine on the developed force.
2.9. Human bronchial smooth muscle cell isolation and culture
Human bronchi were obtained as described above for use in human bronchi isolation. Epithelium was removed by gently rubbing the segments with a pipe cleaner moistened with HBSS. Primary smooth muscle cultures were prepared as previously reported (Kannan et al., 1997). All cultures were maintained in a humidified atmosphere of 5% CO2 and 95% air at 37°C. Upon reaching 70-90% confluence, cells were passaged and plated for use in experiments. Cells were used between passage 2 and 5 for all experiments. Presence of smooth muscle cells were confirmed by immunohistochemistry.
2.10. Human smooth muscle cell treatment
At time of passage, smooth muscle cells were plated onto 8 well borosillicate coverglass chambers and maintained in DMEM/F12 containing 10% FBS and antibiotic/antimycotic. At approximately 50-60% confluence the medium was replaced with serum free medium consisting of DMEM/F12 with 1 mg/ml insulin, 0.67 μg/ml selenium, 0.55 mg/ml transferrin and antibiotic/antimycotic. Durations of this treatment greater than 72 h results in cells that have a previously determined contractile phenotype (Halayko et al., 1999). After 72 h the cells were used for Ca2+ imaging experiments.
2.11. Digital Video Fluorescence Imaging
To measure [Ca2+]i, cells plated in 8 well borosillicate coverglass chambers as described above were incubated with 5 μM Fura-2AM in HBSS for 60 min at room temperature. Cells were then washed twice with fresh HBSS and subsequently maintained in HBSS. Cells were continuously perfused during the acquisition of all Ca2+ measurements. Fluorescence excitation, image acquisition, and Ca2+ data analyses were controlled using a dedicated video fluorescence imaging system (Metafluor; Universal Imaging Corporation). Cells were imaged using an inverted Nikon Diaphot microscope equipped with a Nikon Fluor X20 objective lens. Fura 2-loaded cells were alternately excited at 340 and 380 nm using a Lambda 10-2 filter changer (Sutter Instrument Company). Fluorescence emissions were collected separately for each wavelength using a 510 nm barrier filter. Images were acquired using a Micromax 12 bit camera system (Princeton Instruments) approximately every 0.75 s. [Ca2+]i was calculated from the ratio of intensities at 340 nm and 380 nm, by extrapolation from a calibration curve as previously described (White et al., 2003).
2.12. Materials
DMEM, antibiotic/antimycotic mixture and Insulin-Transferrin-Selenium were obtained from Gibco-Invitrogen, Carlsbad, CA. Indomethacin, AA-861, pyrilamine, isoproterenol, atropine, lanthanum chloride, nifedipine, acetylcholine chloride were purchased from Sigma-Aldrich, St. Louis, MO. BAPTA-AM and pluronic acids were purchased from Molecular Probes, Inc. ω-Conotoxin GVIA and Fura-2 AM were purchased from Calbiochem, La Jolla, CA.
2.13. Data analysis
In all experiment, differences between control and treated groups were analyzed for statistical significance using a one way analysis of variance and Student's t–test (two-tailed) for unpaired sample. In the case of ANOVA a multiple comparison test was used to compare all groups. A vale of P ≤ 0.05 was accepted as significant. Results were expressed as mean ± SE.
3. Results
3.1. Determination of optimal forceresting
Optimal forceresting was operationally defined as the arbitrary preload force applied, in the absence of IF, at which subsequent contraction by 60 mM KCl-substituted Ca2+ buffer elicited a maximal active force (Fig. 1). We determined that an applied force of 4.9 mN (0.5 g) elicited a maximal active force that was not significantly different from those obtained at lesser, and higher, applied forces. Based upon this result, a forceresting of 4.9 mN (0.5 g) was used in all subsequent studies.
Figure 1. Determination of optimal forceresting.
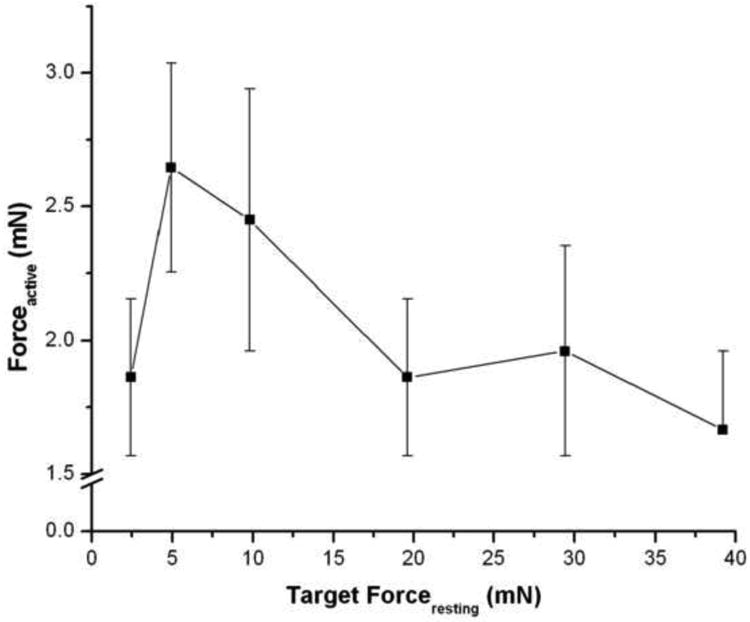
Human bronchi were placed in 0.0 mM Ca2+ buffer. Following equilibration, contraction was elicited by 60 mM KCl in 2.39 mM Ca2+ buffer at various applied resting forces. A forceresting of 4.9 mN (0.5 g) elicited maximal Forceactive and did not differ significantly from lesser, or greater, applied resting forces. The values are the mean ± SE, P > 0.1, n = 5.
3.2. Initial observation of extracellular Ca2+-dependent IF, ACh-elicited force and total force generation
We observed that in airways equilibrated to an optimal (vide supra) applied forceresting of 4.9 mN (0.5 g) in Ca2+-containing PSS that there was an immediate and rapid loss of forceresting upon exchange to a nominally zero Ca2+ PSS (Fig. 2). The magnitude of the dissipated force (i.e., IF) was 1.67 ± 0.39 mN (0.17 ± 0.04 g). IF was immediately restored, and not different from basal values, following the re-addition of Ca2+-containing buffer 1.76 ± 0.39 mN (0.18 ± 0.04 g). ACh (10-5 M) elicited an active force of 1.96 ± 0.29 mN (0.20 ± 0.03 g). The total force, defined as the sum of IF and ACh-elicited force, was 3.72 ± 0.88 mN (0.38 ± 0.09 g). Thus, the IF was approximately half (51.5 ± 7.3 %) of the total airway force generation.
Figure 2. Determination of IF, ACh-elicited force, and total force generation.
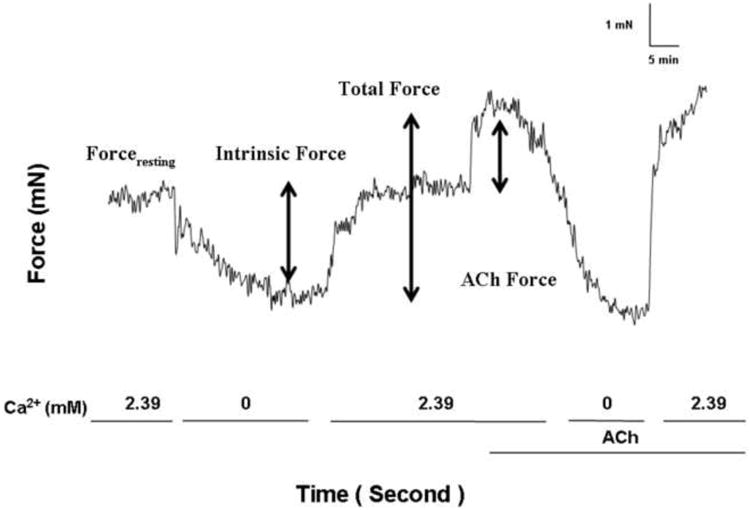
Forceresting was initially set to 4.9 mN (0.5 g) in 2.39 mM Ca2+ buffer. Following buffer substitution with 0.0 mM Ca2+ buffer Forceresting decreased to a stable nadir value. IF was defined as the difference between initial force and the nadir value. Subsequently, the buffer was replaced with 2.39 mM Ca2+ buffer and forceresting was not significantly different than original. ACh 10-5 M was added (ACh-elicited force). Total force was the sum of IF and ACh-elicited force.
3.3 Effect of epithelium on IF in human bronchi
Mechanical removal of epithelium, which was confirmed using H&E staining and phase contrast microscopy, did not significantly alter the magnitude of IF (data not shown). Thus, in subsequent pharmacologic studies only airways with intact epithelium were studied.
3.4. Concentration dependent effect of Ca2+ addition on IF
Small human airways have an IF which is rapidly revealed by the removal of extracellular Ca2+. Figure 3 shows that the sequential re-addition of Ca2+ results in the generation of force upon each stepwise increase in extracellular Ca2+ from 0.0 to 10.0 mM Ca2+ was not quantal, but occurred in a concentration dependent manner.
Figure 3. Representative tracing of the influence of extracellular Ca2+ concentration on IF.
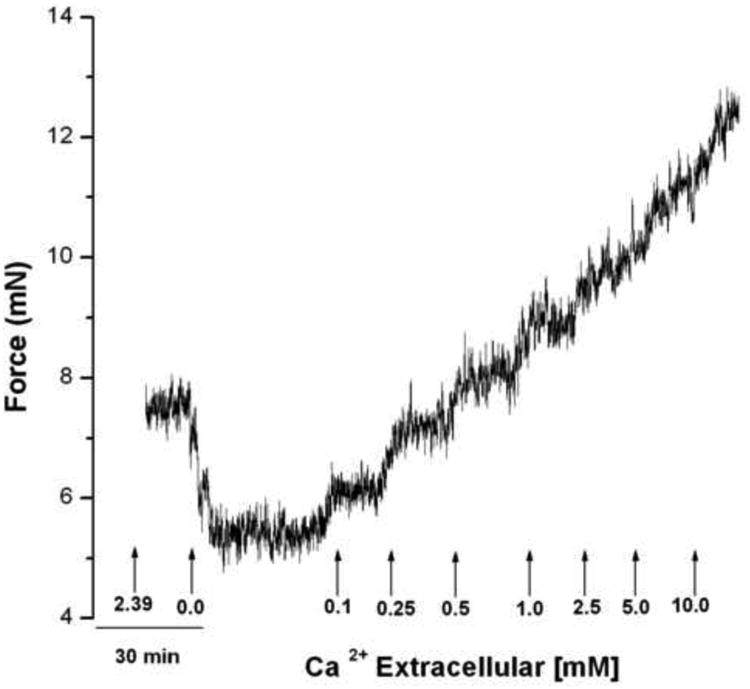
Human bronchi rings were placed in 2.39 mM Ca2+ buffer and the tension was set to 4.9 mN (0.5 g). After 1 hour equilibration, the buffer was rapidly replaced by 0 CaPSS resulting in rapid relaxation; this was maintained for at least 30 min at which time Ca2+ was sequentially added by substitution with buffer containing 0.1, 0.25, 0.5, 1.0, 2.5, 5.0 or 10.0 mM Ca2+. Each concentration was maintained for 20 min to attain a stable force level. Observe the graded contraction produced at each sequential Ca2+ addition (n = 3).
3.5. Effect of lanthanum on IF
The addition of 1 mM lanthanum, a non-specific cation channel blocker, to epithelium-intact human bronchi did not inhibit the ability of nominally zero Ca2+ buffer to decrease forceresting, indicating the continued presence of IF (Fig. 4). Interestingly, addition of 1 mM lanthanum to human bronchi in Ca2+ containing buffer (2.39 mM) prevented resting force decrease by 70.6 ± 3.8 % compared to 0.0 mM Ca2+. These results suggest that La3+ effect on inhibiting Ca2+ influx influences IF more than its La3+ effect on Ca2+ efflux.
Figure 4. Influence of Lanthanum on IF of epithelial-intact human bronchi.
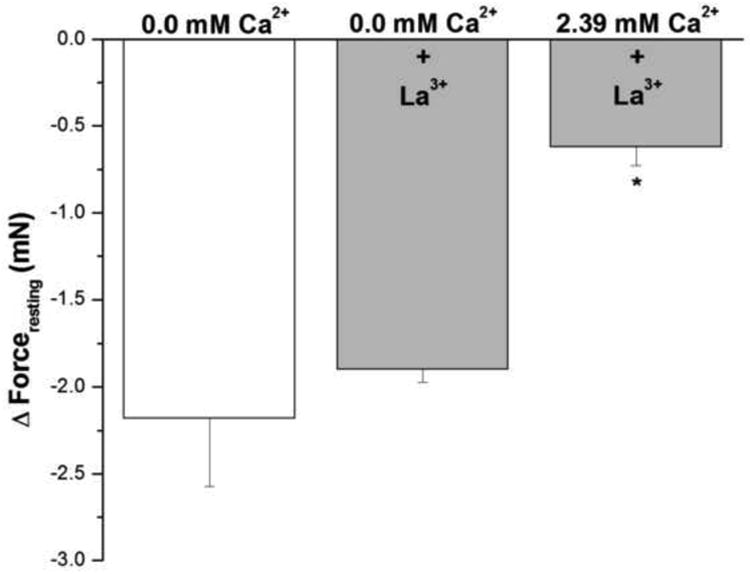
IF was not different in epithelium intact human bronchi determined following exposure to Ca2+ free solution (0.0 mM Ca2+) when compared to the addition of lanthanum (1 mM) in nominally zero Ca2+ - buffer. However, in the presence of 2.39 mM Ca2+ 1 mM lanthanum did not dissipate IF. * P < 0.01 compared with control. Data were the mean ± SE. Mean of 6 experiments, with tissue from 6 individuals. Zero Ca2+ buffer, Zero Ca2+ buffer containing 1 mM lanthanum and PSS buffer with 1 mM lanthanum resulted in a significant decrease in PSS buffer basal tone in ASM (P < 0.001 and P < 0.01, respectively, data not shown).
3.6. Effect of zero [Ca2+]-buffer on [Ca2+]i in cultured human airway myocytes and epithelium intact human bronchi
In cultured airway myocytes [Ca2+]i was determined. Following the acute replacement of 2.25 mM extracellular [Ca2+]-buffer with zero [Ca2+]-buffer [Ca2+]i decreased significantly from 90.1 ± 2.2 to 69.4 ± 0.9 nM. P < 0.001 (results are mean ± SE, n=6 experiments, at least 50 individual cells analyzed per experiment). Upon reexchange of zero [Ca2+]-buffer with 2.25 mM [Ca2+]-buffer [Ca2+]i was not different from initial values.
In Fura-2-loaded epithelium-intact bronchial smooth muscle strips superfused in vitro; the F340/F380 ratio was monitored continuously and used as an indicator of relative changes in [Ca2+]i levels. Initially, each strip was equilibrated with 2.39 mM extracellular Ca2+, giving an average ratio of 0.47 ± 0.04 (mean ± SE, n = 4). Rapid switching to Ca2+-free medium resulted in a significant decrease in [Ca2+]i (0.33 ± 0.05). The levels were restored to initial value (0.44 ± 0.04) upon re-introduction of Ca2+ to the medium (Figs 5A and 5B).
Figure 5. Effects of removal of extracellular Ca2+ on intracellular Ca2+ in intact human bronchi.
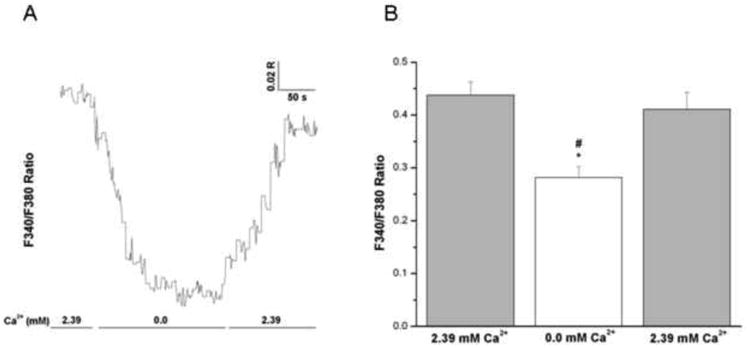
(A) Representative trace showing the effect of removal and reintroduction of extracellular Ca2+ on global [Ca2+]i levels in an intact human bronchial segment superfused in vitro. Notice the decrease in 340/380 ratio upon removal of extracellular Ca2+ and the subsequent increase in the 340/380 ratio upon the reintroduction of extracellular Ca2+. Similar results were obtained in 4 separate experiments. (B) Histogram showing the average of all experiments demonstrating a significant decrease in global [Ca2+]i levels correspondent with removal of Ca2+ from the extracellular medium in intact human bronchi, * P < 0.01. Upon reintroduction of extracellular Ca2+ there is a significant increase in global [Ca2+]i when compared to removal of Ca2+ from the extracellular medium, # P < 0.05. Data are represented as mean ± SE. Mean of 4 experiments, with tissue from 4 individuals.
3.7. Effect of BAPTA–AM on IF in epithelium intact human bronchi
Figure 6 shows the effect of the cell permeant Ca2+ chelating agent BAPTA–AM on forceresting in epithelium intact human bronchi. Lower concentrations of BAPTA–AM (10 μM and 20 μM) reduced IF similar to that observed in nominally zero Ca2+- buffer. There was an even greater decrease in IF of the isolated human bronchi at the highest concentration of BAPTA–AM (100 μM), * P < 0.05 when compared with removal of extracellular Ca2+.
Figure 6. Effect of BAPTA–AM on IF in epithelium intact human bronchi.
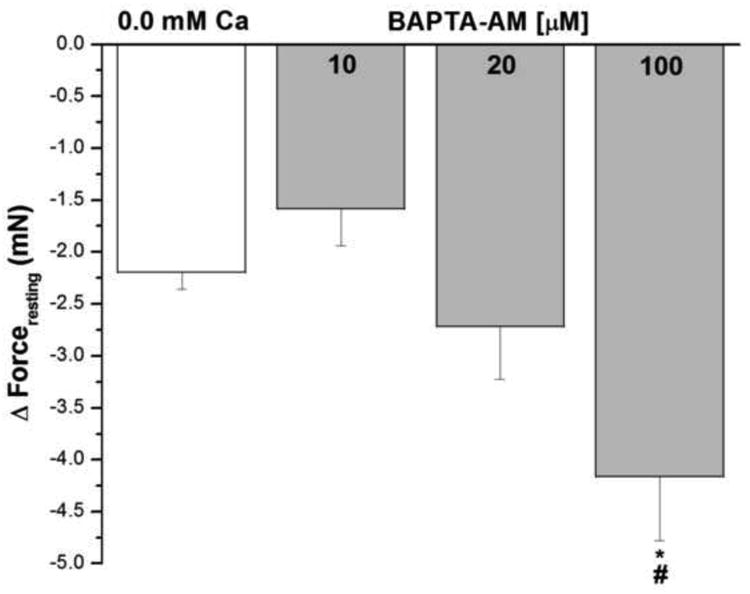
The forceresting of the isolated human bronchi was significant decreased by higher concentration BAPTA–AM (100 μM), *, P < 0.05 compared with control. Lower concentration BAPTA–AM (10 μM and 20 μM) reduced IF similar to nominally zero Ca2+- buffer. BAPTA–AM 100 μM vs. 10 μM, # P < 0.05. Data were the mean ± SE. Mean of 4 experiments, with tissue from 4 individuals. Zero Ca2+ buffer or PSS containing 10 μM, 20 μM and 100 μM BAPTA–AM caused a significant fall in PSS buffer basal tone in ASM (P < 0.001 and P < 0.01, respectively, data not shown).
3.8. Effect of isoproterenol on IF and basal [Ca2+]i nM in human airway smooth muscle cells
Addition of isoproterenol in concentrations of 10-8 M to 10-5 M (Fig. 7A) reduced IF similar to nominally zero Ca2+- buffer. The acute addition of isoproterenol at concentrations of 10-9, 10-8 or 10-7 M for 5 min did not alter basal [Ca2+]i. However, exposure of HBSMCs to a concentration of 10-6 M isoproterenol resulted in a significant decrease in basal [Ca2+]i (from 104.2 ± 1.07 to 98.43 ± 1.41 nM, Fig. 7B) compared with cells exposed to HBSS (Control).
Figure 7. Influence of β–adrenergic agonist on IF of epithelial-intact human bronchi and on basal [Ca2+]i in human airway smooth muscle cells.
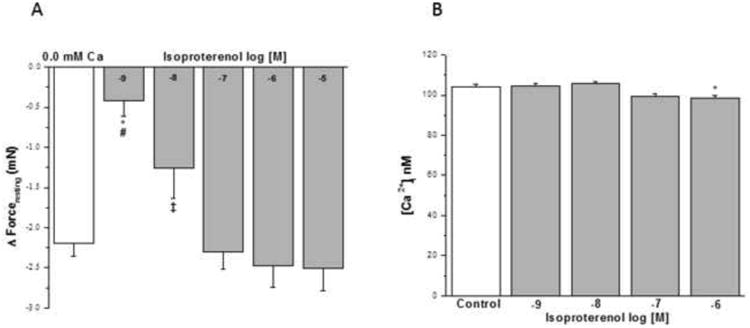
IF in human bronchi determined following exposure to Ca2+ free solution was compared the IF elicited by the acute addition of isoproterenol. Isoproterenol at concentration of 10-8 M to 10-5 M reduced IF. * P < 0.01 compared with control; # P < 0.01 isoproterenol 10-9 M vs. 10-5 M, 10-6 M and 10-7 M; ‡ P < 0.05 isoproterenol 10-8 M vs. 10-5 M (Fig. 7A). Data were the mean ± SE. Mean of 4 experiments, with tissue from 4 individuals. PSS containing 10-8 to 10-5 M isoproterenol had a significant decrease in level of PSS buffer basal tone in ASM (P < 0.01 and P < 0.001, respectively, data not shown). However, PSS buffer with 10-9 M isoproterenol had no significant effect on level of PSS buffer basal tone in ASM (Data not shown). However, the acute addition of isoproterenol at concentrations of 10-9 to 10-7 M for 5 min did not alter basal [Ca2+]i in HBSMCs. However, concentrations of isoproterenol at 10-6 M resulted in a significant decrease in basal [Ca2+]i compared with HBSMC exposed to HBSS (Control), isoproterenol 10-9 M and 10-8 M, * P < 0.05 (results are mean ± SE, n = 4-8 experiments, at least 50 individual cells analyzed per experiment) (Fig. 7B).
3.9. Effect of atropine on IF and basal [Ca2+]i nM in human airway smooth muscle cells
Atropine from 10-9 to 10-6 M (Fig. 8A) had a negligible effect on IF. Concentrations ≥10-5 M had a small effect on reducing IF (~ 1/3). However, the acute addition of HBSS containing 10-9- 10-4 M atropine to HBSMC had no significant effect on basal [Ca2+]i (Fig. 8B).
Figure 8. Influence of muscarinic antagonist on IF of epithelial-intact human bronchi and basal [Ca2+]i in human airway smooth muscle cells.
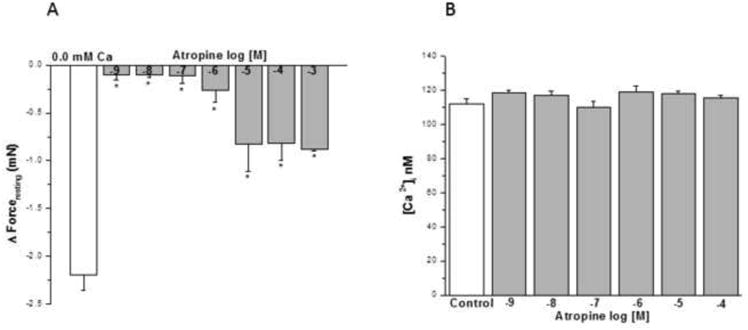
Atropine showed no effect on IF at concentration of 10-9 M to 10-3 M, * P < 0.01 compared with control (Fig. 8A). Data were the mean ± SE. Mean of 4 experiments, with tissue from 4 individuals. PSS containing 10-4 or 10-3 M atropine resulted in a significant decrease in PSS buffer basal tone in ASM (P < 0.01 and P < 0.001, respectively, data not shown). However, PSS buffer with 10-9 to10-5 M atropine had no significant effect on PSS buffer basal tone in ASM (Data not shown). However, the acute addition of atropine (10-9 to 10-4 M) to HBSMCs resulted in no significant changes in basal [Ca2+]i (results are mean ± SE, n = 3 experiments, at least 50 individual cells analyzed per experiment) (Fig. 8B).
3.10. Effect of nifedipine on IF, basal [Ca2+]i nM in human airway smooth muscle cells and KCL induced contraction in epithelium intact human bronchi
Nifedipine at greater than dihydropyridine selective concentrations, 10-7 M to 10-5 M, reduced forceresting similar to nominally zero Ca2+-buffer (Fig. 9A). The acute addition of HBSS containing 10-9 to 10-7 M nifedipine to HBSMC for 8 min had no significant effects on basal [Ca2+]i compared with controls (Fig. 9B). However, 10-6 M nifedipine induced a rapid and significant increase in [Ca2+]i compared with controls (198.6 ± 11.40 nM vs 115.6 ± 2.84 nM, Fig. 9B). Also 10-5 M nifedipine induced a significant increase in [Ca2+]i compared with control (621.5 ± 24.02 nM vs 115.6 ± 2.84 nM, Fig. 9B).
Figure 9. Influence of dihydropyridine antagonist on IF of epithelial-intact human bronchi, basal [Ca2+]i in human airway smooth muscle cells and KCL induced contraction in intact human bronchi.
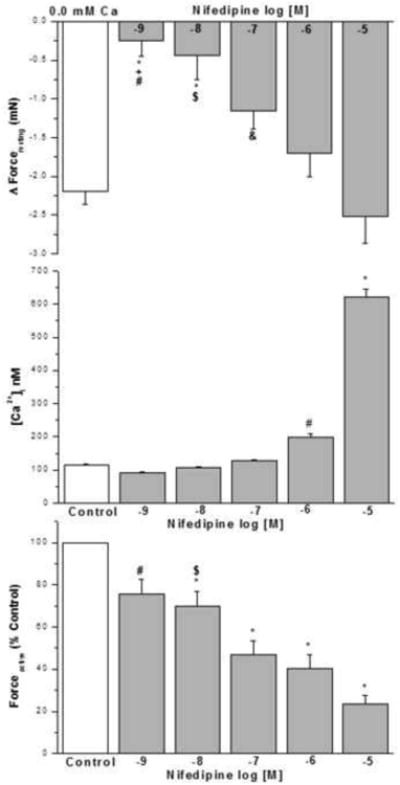
Low concentration nifedipine (10-9 M and 10-8 M) showed no effect on the IF, higher concentration (10-7 M to 10-5 M) relaxed the isolated human bronchi. * P < 0.01 compared with control. Nifedipine 10-5 M vs. 10-9 M, 10-8 M and 10-7 M, # P < 0.001; $ P < 0.01 and & P < 0.05, respectively. Nifedipine 10-6 M vs. 10-9 M, † P < 0.05 (Fig. 9A). N = 4-6 each group, each column represents the mean ± SE. PSS containing 10-7 to 10-5 M nifedipine caused in a significant decrease in PSS buffer basal tone in ASM (P < 0.01 and P < 0.001, respectively, data not shown).
The acute addition of nifedipine at 10-9 to 10-7 M resulted in no significant effect on basal [Ca2+]i when compared to controls. The acute addition of 10-5 M nifedipine for 8 min, induced a rapid and significant increase in [Ca2+]i when compared to controls, * P < 0.001 or when compared to HBSMCs exposed to 10-9 to 10-6 M nifedipine, also 10-6 nifedipine for 8 min, induced a rapid and significant increase in [Ca2+]i when compared to controls, # P < 0.001 or when compared to HBSMCs exposed to 10-9 to 10-7 M nifedipine(results are mean ± SE, n =5-6 experiments, at least 50 individual cells analyzed per experiment) (Fig. 9B).
This experiment examines the effect of nifedipine on KCl induced contraction. An initial contraction (K1, in the absence of nifedipine) was compared to a subsequent contraction in the absence (Control) or presence of nifedipine at 10-9 to 10-5 M (K2). Atropine (10-6 M) was present all experiments to eliminate the effects of ACh release from nerve termini. Nifedipine decreased the KCl-elicited force in a concentration dependent manner. Data were calculated by dividing K2 by K1 and then converting to percent control. The IC 50 is ~ 10-7 M. Data are mean ± SE, with tissue from 6 individuals, * P < 0.001 compared with control; # P < 0.05 nifedipine 10-9 M vs. 10-7 M, 10-6 M and 10-5 M; $ P < 0.05 nifedipine 10-8 M vs. 10-6 M and 10-5 M (Fig. 9C).
This set of experiments examined the effect of nifedipine on KCl-induced contraction. We compare an initial contraction (K1) to a 2nd subsequent contraction (K2) in the absence (control) or presence of nifedipine (10-9 to 10-5 M). These experiments were performed in the presence of atropine 10-6 M to eliminate the contribution of acetylcholine release from nerve termini. In epithelium intact human bronchi the addition of nifedipine at all concentrations led to a significant reduction in the ratio of K2/K1 (Fig. 9C). Nifedipine decreases KCl-elicited force in a concentration dependent manner. The IC 50 is ~ 10-7 M. (These experiments were done to show that the effects of nifedipine on VOC occur at concentrations much lesser than the concentrations which reduce IF.)
3.11. Effect of indomethacin, AA-861 and pyrilamine on IF in epithelium intact human bronchi
We examined the effect of cyclo-oxygenase inhibitor (indomethacin, 10 μM); 5-lipoxygenase inhibitor (AA-861, 1 μM); histamine H-1 antagonist (pyrilamine, 10 μM); and the combination of the cyclo-oxygenase inhibitor plus 5-lipoxygenase inhibitor (indomethacin 10 μM + AA-861 1μM). As shown in figure 10, IF of small human airway smooth muscle was not significantly affected by indomethacin, AA-861 or pyrilamine either alone or in combination. However, as shown in figure 10, both indomethacin and AA-861 reduced ACh–elicited force in human airway smooth muscle, whereas pyrilamine had no effect. However, when total force is examined, the influence of indomethacin, AA-861 alone or in combination was no longer evident.
Figure 10. Effect of cyclooxygenase, leukotrienes, and histamine on IF and ACh-elicted force generation of epithelial-intact human bronchi.
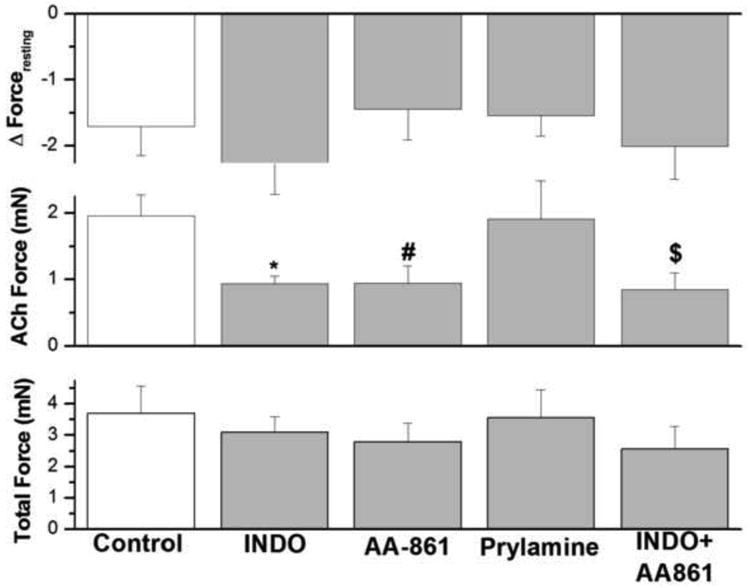
IF in human bronchi determined following and during the continued presence of indomethacin, AA-861, pyrilamine, and the combination of indomethacin and AA-861 did not affect IF. However, indomethacin, AA-861 and the combination of indomethacin and AA-861 significantly decreased ACh-elicited force generation, *P < 0.01; # P < 0.05; $ P < 0.05, respectively compared with control. There is no significant difference in total force between the different groups. Data are the mean ± SE. Mean of 17 experiments, with tissue from 17 individuals. PSS containing 1 μM AA-861 or 10 μM pyrilamine resulted in a significant decrease in PSS buffer basal tone in ASM (P < 0.01, Data not shown). However, PSS buffer with 10 μM indomethacin had no significant effect on PSS buffer basal tone in ASM (Data not shown).
3.12. Effect of ω-conotoxin on IF in epithelium intact human bronchi
Figure 11 shows the effect of ω-conotoxin, an antagonist of voltage– activated N–type Ca2+ channels and of neurotransmitter release at neuronal synapses, on IF in epithelium intact human bronchi. Conotoxin showed no effect on the forceresting, i.e. the IF of isolated human bronchi was not significantly decreased by 10-6 M conotoxin.
Figure 11. Effect of conotoxin on IF in epithelium intact human bronchi.
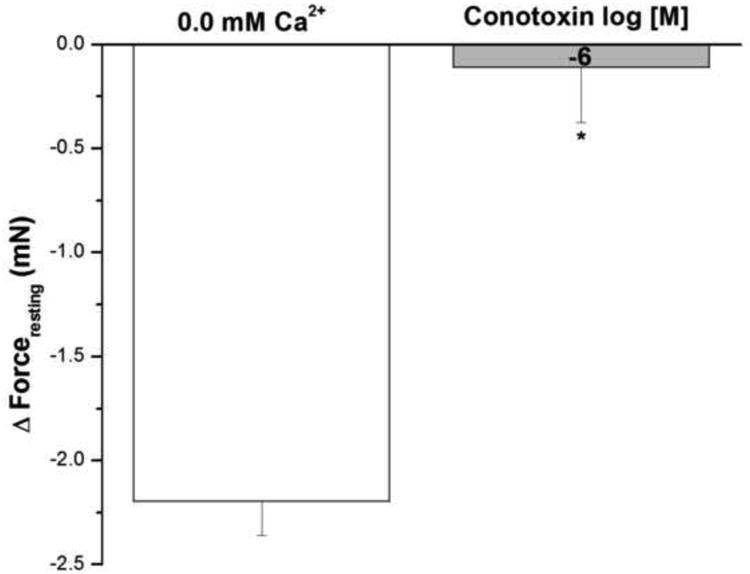
Conotoxin showed no effect on IF, the IF of isolated human bronchi was significant decreased by 10-6 M conotoxin, * P < 0.001 compared with control. Data were the mean ± SE, with tissue from 3 individuals. PSS buffer with 10-6 M conotoxin had no significant effect on PSS buffer basal tone in ASM (Data not shown).
4. Discussion
Although the existence of IF in human small airways has been previously demonstrated in vitro (Rabe et al., 1993), the magnitude and regulation of IF has not been studied systematically. Furthermore, results of prior studies that examined the roles and interrelationships of cysteinyl–leukotrienes (Ellis and Undem, 1994), histamine (Hutas et al., 1981), cyclooxygenase products (Ito et al., 1989), extracellular Ca2+ (Bengtsson et al., 1987), and dihydropyridine calcium (Ca2+) channel antagonist on IF in human ASM have been contradictory.
We operationally define IF as the presence of inherent smooth muscle force generation in the absence of known concurrent exogenous stimulation. In our study IF was identified by reducing the extracellular [Ca2+] to nominally zero in isometrically mounted 2 mm human bronchi.
We found that the IF of 2 mm diameter unstimulated in vitro human airways was epithelial independent and was approximately equal in magnitude to the maximal active force generation elicited by ACh (10-5 M). Specifically, when normalized to the total force generation of each airway, the IF represents 51.5 ± 7.3 % of the total airway force generation. This value is nearly identical to the 46 ± 25% (mean ± SD) determined by Davis et al (Davis et al., 1982) in tracheal to 2nd order bronchi following addition of EDTA to human airway strips. Likewise, our results are similar to Taylor et al (Taylor et al., 1984) who found that maximal resting tone in segmental and subsegmental airways strips could be increased above baseline 1,460 ± 170 mg by carbachol whereas it could be reduced below baseline 980 ± 98 mg by theophylline as well as Ellis et al (Ellis and Undem, 1994) who quantified IF as 65% ± 9% of maximal tone elicited by barium contraction compared to maximal relaxation elicited by isoproterenol in fresh tissue but only 31 ± 6% in overnight incubated surgically obtained 3-12 mm human bronchi. Thus, the unstimulated airway in vitro “sits” about ½ maximally contracted compared to the “fully” relaxed state when IF is determined by reducing the extracellular calcium concentration to nominally zero. Our results differ from the study of Davis et al (Davis et al., 1982) in that they found significant variance amongst specimens from tracheal and first-order bronchial smooth muscle likely due to many tissues being taken from cadavers compared to our exclusive use of fresh surgical discard. This property of unstimulated airway smooth muscle to have ½ maximal activation of the contractile apparatus in the unstimulated condition is different from canine (Cabezas et al., 1971), bovine (Zhao and Guenard, 1995), ovine (Bosse et al., 2009), feline (Altiere et al., 1984) and porcine trachealis (Croxton et al., 1995) but similar to guinea pig (Bard et al., 1998).
As we found that removal of extracellular Ca2+ revealed the existence of IF we also noted that the re-addition of extracellular calcium restored IF in a concentration-dependent manner. Our findings identified a permissive relationship between extracellular Ca2+ and force generation that was insensitive to La+3 sensitive Ca2+ efflux or influx channels. In our study simultaneous force and Fura-2 determined intracellular [Ca2+] clearly indicate that IF elicited by removal of extracellular Ca2+ reduces cytoplasmic intracellular calcium. In addition, in isolated myocytes changing the extracellular Ca2+ concentration from 2.25 mM to zero reduced the cytoplasmic [Ca2+] from 90.1 ± 2.2 nM (mean ± SE) to 69.4 ± 0.9 nM (mean ± SE). Likewise chelation of intracellular Ca2+ with BAPTA in unstimulated airways reveals the presence of IF. Not surprisingly these findings suggest that [Ca2+] directly effects IF and is influenced by a permissive extracellular Ca2+ influx. The Ca2+ influx was not due to La+3 sensitive channels.
IF also became apparent following the addition of isoproterenol in an intracellular Ca2+-independent manner. This finding is consistent with a decrease in unstimulated force in isolated human bronchi following addition of β–adrenoreceptor agonists (Nials et al., 1993). The effects of isoproterenol were likely not due to effects on calcium mobilization as it produced no effect on basal intracellular Ca2+ in isolated human airway myocytes. As Rho kinase phosphorylates myosin light chain phosphatase decreasing its activity there is a greater accumulation of phosphorylated myosin for any given intracellular calcium and thus a change in Ca2+ sensitivity (Janssen et al., 2004). Isoproterenol suppresses RhoA and Rho kinase activates as well as directly suppressing myosin light chain kinase activity. The effect of isoproterenol to reveal IF suggests that in unstimulated human airway smooth muscle intracellular [Ca2+] is sufficient to promote a constitutive myosin light chain kinase activity as has been shown by (Ammit et al., 2000).
IF was not completely due to in vivo cholinergic neuronal release of ACh as the maximal effect of the cholinergic receptor antagonist atropine on IF was about 1/3rd that due to zero extracellular Ca2+. In addition, ω-conotoxin an N-type Ca2+ channel blocker which inhibits the Ca2+-dependent release of acetylcholine and neuropeptides (Altiere et al., 1992) did not effect IF. These findings suggest that IF is due to a primary ASM property rather than a neural effect.
The role of membrane voltage gated Ca2+ channels on IF is confusing. Often others including Ito et al (Ito et al., 1985) infer VOCs as casual to IF having used non pharmacologic L-type specific concentrations of verapamil (30 μM) which reduced human airway smooth muscle Ca2+-induced contraction by ~ 40%. Interestingly, nifedipine at higher than micromolar concentrations was able to relax IF. In our experiments 100 nM elicited ½ maximal relaxation of IF. Whether nifedipine was acting exclusively via L-type dihydropyridine channels or not was not determined. In our studies the IC50 for nifedipine against KCl contraction was 100 nM which is much greater than the ~10 nM published IC50 for this agent as a “selective” L-type Ca2+ channel blocker (Janssen et al., 2004). Indeed, Janssen et al (Janssen, 1997), and others (Yamakage et al., 2001) have shown previously that nifedipine at higher concentrations can partially inhibit T-type Ca2+ channels as well as other nonselective channels. As reported by others the complex effects of nifedipine used to determine IF determination were noted by the paradoxical increases in intracellular calcium concentration at higher concentrations of nifedipine.
To clarify the effect of prostanoids, AA, and histamine on IF we examined the effect of indomethacin (cyclooxygenase inhibitor), AA-861 (5-lipoxygenase inhibitor), and pyrilamine (antihistamine antagonist) on IF and ACh-elicited active force. Though indomethacin and AA-861 diminished ACh force, neither agent nor pyrilamine effected IF.
The effects of indomethacin on the IF of human airway smooth muscle are the subject of some debate. Some results have shown that increases (Coleman et al., 1996), reduction (Ito et al., 1989) or no effect of indomethacin on IF (Brink et al., 1980). Likewise previous reports indicate that AA–861 and pyrilamine both have little or no effect on human airway IF (Ellis and Undem, 1994).
Finally, our study is similar to a prior study by Watson et al. and finds the influence of the bronchial epithelium on human airway IF and active tension to be very limited (Watson et al., 1997). This result differs from guinea pig trachea where spontaneous force has been clearly determined to depend upon constitutively derived airway epithelial prostaglandin synthesis (Orehek et al., 1975).
We speculate that an increase of IF, a fundamental property of ASM, could be related to human airway clinical hyperresponsiveness, i.e. asthmatic tendency. Furthermore our data supports the speculation of others (Montano and Bazan-Perkins, 2005) that unstimulated Ca2+ entry contributes to resting intracellular [Ca2+] and influences cross-bridge cycling rates and hence IF in ASM. For example, Fox et al (Fox and Daniel, 1979) found that basal active tension of smooth muscle in the lower esophageal sphincter of the opossum was due to the inward leak of Ca2+, in that case blocked by verapamil.
Some suggest that in vivo healthy lungs have only negligible IF as there is only a minimal influence of bronchodilators on maximal expiratory flow suggesting little inherent smooth muscle contractile force. However, the relationship between lung volume and inducible bronchomotor tone in normal subjects (i.e., as determined by the reduction of methacholine-elicited bronchoconstriction following deep inspiration) is consistent with ASM IF. These observations suggest both the existence of IF of ASM in healthy subjects and its increase in disease states such as asthma (Fish et al., 1981). Moreover, IF becomes more apparent at lower lung volumes as notable increases in expiratory airflow occur in normal subjects following aerosolized isoproterenol, but only at lower lung volumes (50 and 25% vital capacity) (O'Donnell et al., 1986). Finally, IF of human airways may contribute to a significant portion of total lung elastic recoil (Crawford et al., 1987).
In this study we find that intracellular Ca2+ is the likely regulator of IF. This effect is not due to epithelial influence, active neurotransmitter release nor ongoing prostanoid, nor arachidonate metabolism. As intracellular Ca2+ chelation relaxed unstimulated airway and as zero extracellular Ca2+ rapidly reduced intracellular calcium a permissive relationship between extracellular Ca2+, intracellular Ca2+ and IF exists. We (Sieck et al., 2008; White et al., 2006) and others (Kang et al., 2005; Vazquez et al., 2003) have previously shown that store-operated calcium entry (SOCE) release is influenced by TRPC and CD38 expression. Moreover, other human tissues (Wang et al., 2003) with spontaneous tonic contraction, such as the esophagus, uniquely have enhanced capacitative or store-operated calcium entry (SOCE) due to TRPC3. We speculate that in inflammatory airway diseases (asthma/COPD) TNF-mediated TRPC3/CD38 enhanced SOCE (Prakash et al., 1998) may increase IF.
Intrinsic force generation is a fundamental property of human airway smooth muscle. > Intrinsic force can be identified by reducing extracellular calcium concentration in isolated isometrically mounted 2 mm human bronchi. > The magnitude of intrinsic force is ~ 50% of maximal acetylcholine elicited force. > There is a permissive relationship between extracellular Ca2+, [Ca2+]i and IF.
Acknowledgments
We thank the division of anatomic pathology, department of laboratory medicine and pathology staff for help collecting specimen. This work was supported by National Institute of Health grant AI050494 and HL74309.
Footnotes
Authorship contributions: MEW participated in experimental design, review and interpretation of data, preparation of the manuscript. AX performed all experiment, data analysis and wrote manuscript draft. GCS contributed to the initial study design, interpretation of results and editing the manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Altiere RJ, Diamond L, Thompson DC. Omega-conotoxin-sensitive calcium channels modulate autonomic neurotransmission in guinea pig airways. The Journal of pharmacology and experimental therapeutics. 1992;260:98–103. [PubMed] [Google Scholar]
- Altiere RJ, Szarek JL, Diamond L. Neural control of relaxation in cat airways smooth muscle. Journal of applied physiology: respiratory, environmental and exercise physiology. 1984;57:1536–1544. doi: 10.1152/jappl.1984.57.5.1536. [DOI] [PubMed] [Google Scholar]
- Ammit AJ, Armour CL, Black JL. Smooth-muscle myosin light-chain kinase content is increased in human sensitized airways. American journal of respiratory and critical care medicine. 2000;161:257–263. doi: 10.1164/ajrccm.161.1.9901005. [DOI] [PubMed] [Google Scholar]
- Bard M, Salmeron S, Coirault C, Blanc FX, Lecarpentier Y. Effects of initial length on intrinsic tone in guinea pig tracheal smooth muscle. The American journal of physiology. 1998;275:L1026–1030. doi: 10.1152/ajplung.1998.275.6.L1026. [DOI] [PubMed] [Google Scholar]
- Bengtsson B, Khan AR, Weiber R. Low potency of Ca antagonists in smooth muscle from different levels of the respiratory tract. Acta Physiologica Scandinavica. 1987;131:249–256. doi: 10.1111/j.1748-1716.1987.tb08234.x. [DOI] [PubMed] [Google Scholar]
- Bosse Y, Chin LY, Pare PD, Seow CY. Adaptation of airway smooth muscle to basal tone: relevance to airway hyperresponsiveness. American journal of respiratory cell and molecular biology. 2009;40:13–18. doi: 10.1165/rcmb.2008-0150OC. [DOI] [PubMed] [Google Scholar]
- Bosse Y, Sobieszek A, Pare PD, Seow CY. Length adaptation of airway smooth muscle. Proceedings of the American Thoracic Society. 2007;5:62–67. doi: 10.1513/pats.200705-056VS. [DOI] [PubMed] [Google Scholar]
- Brink C, Grimaud C, Guillot C, Orehek J. The interaction between indomethacin and contractile agents on human isolated airway muscle. British journal of pharmacology. 1980;69:383–388. doi: 10.1111/j.1476-5381.1980.tb07026.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabezas GA, Graf PD, Nadel JA. Sympathetic versus parasympathetic nervous regulation of airways in dogs. Journal of applied physiology: respiratory, environmental and exercise physiology. 1971;31:651–655. doi: 10.1152/jappl.1971.31.5.651. [DOI] [PubMed] [Google Scholar]
- Coleman RA, Nials AT, Rabe KF, Vardey CJ, Watson N. Isolated, electrically-stimulated airway preparations--their use in determining beta-adrenoceptor agonist activity. Pulmonary pharmacology. 1996;9:107–117. doi: 10.1006/pulp.1996.0012. [DOI] [PubMed] [Google Scholar]
- Crawford AB, Makowska M, Engel LA. Effect of bronchomotor tone on static mechanical properties of lung and ventilation distribution. Journal of Applied Physiology. 1987;63:2278–2285. doi: 10.1152/jappl.1987.63.6.2278. [DOI] [PubMed] [Google Scholar]
- Croxton TL, Lande B, Hirshman CA. Role of intracellular pH in relaxation of porcine tracheal smooth muscle by respiratory gases. The American journal of physiology. 1995;268:L207–213. doi: 10.1152/ajplung.1995.268.2.L207. [DOI] [PubMed] [Google Scholar]
- Davis C, Kannan MS, Jones TR, Daniel EE. Control of human airway smooth muscle: in vitro studies. Journal of Applied Physiology: Respiratory, Environmental & Exercise Physiology. 1982;53:1080–1087. doi: 10.1152/jappl.1982.53.5.1080. [DOI] [PubMed] [Google Scholar]
- Driver AG, Kukoly CA, Ali S, Mustafa SJ. Adenosine in bronchoalveolar lavage fluid in asthma. The American review of respiratory disease. 1993;148:91–97. doi: 10.1164/ajrccm/148.1.91. [DOI] [PubMed] [Google Scholar]
- Ellis JL, Undem BJ. Role of cysteinyl-leukotrienes and histamine in mediating intrinsic tone in isolated human bronchi. American Journal of Respiratory & Critical Care Medicine. 1994;149:118–122. doi: 10.1164/ajrccm.149.1.8111568. [DOI] [PubMed] [Google Scholar]
- Fish JE, Ankin MG, Kelly JF, Peterman VI. Regulation of bronchomotor tone by lung inflation in asthmatic and nonasthmatic subjects. Journal of Applied Physiology: Respiratory, Environmental & Exercise Physiology. 1981;50:1079–1086. doi: 10.1152/jappl.1981.50.5.1079. [DOI] [PubMed] [Google Scholar]
- Fox JA, Daniel EE. Role of Ca2+ in genesis of lower esophageal sphincter tone and other active contractions. The American journal of physiology. 1979;237:E163–171. doi: 10.1152/ajpendo.1979.237.2.E163. [DOI] [PubMed] [Google Scholar]
- Halayko AJ, Camoretti-Mercado B, Forsythe SM, Vieira JE, Mitchell RW, Wylam ME, Hershenson MB, Solway J. Divergent differentiation paths in airway smooth muscle culture: induction of functionally contractile myocytes. The American journal of physiology. 1999;276:L197–206. doi: 10.1152/ajplung.1999.276.1.L197. [DOI] [PubMed] [Google Scholar]
- Hawkins DF, Schild HO. The Action of Drugs on Isolated Human Bronchial Chains. British journal of pharmacology. 1951;6:682–690. doi: 10.1111/j.1476-5381.1951.tb00680.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogg JC, Chu F, Utokaparch S, Woods R, Elliott WM, Buzatu L, Cherniack RM, Rogers RM, Sciurba FC, Coxson HO, Pare PD. The nature of small-airway obstruction in chronic obstructive pulmonary disease. The New England journal of medicine. 2004;350:2645–2653. doi: 10.1056/NEJMoa032158. [DOI] [PubMed] [Google Scholar]
- Hutas I, Hadhazy P, Debreczeni L, Vizi ES. Relaxation of human isolated bronchial smooth muscle. Role of prostacyclin and prostaglandin F2 alpha in muscle tone. Lung. 1981;159:153–161. doi: 10.1007/BF02713911. [DOI] [PubMed] [Google Scholar]
- Ito M, Baba K, Takagi K, Satake T, Tomita T. Some properties of calcium-induced contraction in the isolated human and guinea-pig tracheal smooth muscle. Respiration Physiology. 1985;59:143–153. doi: 10.1016/0034-5687(85)90003-9. [DOI] [PubMed] [Google Scholar]
- Ito Y, Suzuki H, Aizawa H, Hakoda H, Hirose T. The spontaneous electrical and mechanical activity of human bronchial smooth muscle: its modulation by drugs. British journal of pharmacology. 1989;98:1249–1260. doi: 10.1111/j.1476-5381.1989.tb12671.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janssen LJ. T-type and L-type Ca2+ currents in canine bronchial smooth muscle: characterization and physiological roles. The American journal of physiology. 1997;272:C1757–1765. doi: 10.1152/ajpcell.1997.272.6.C1757. [DOI] [PubMed] [Google Scholar]
- Janssen LJ, Tazzeo T, Zuo J, Pertens E, Keshavjee S. KCl evokes contraction of airway smooth muscle via activation of RhoA and Rho-kinase. American journal of physiology. 2004;287:L852–858. doi: 10.1152/ajplung.00130.2004. [DOI] [PubMed] [Google Scholar]
- Kang BN, Deshpande DA, Tirumurugaan KG, Panettieri RA, Walseth TF, Kannan MS. Adenoviral mediated anti-sense CD38 attenuates TNF-alpha-induced changes in calcium homeostasis of human airway smooth muscle cells. Canadian journal of physiology and pharmacology. 2005;83:799–804. doi: 10.1139/y05-081. [DOI] [PubMed] [Google Scholar]
- Kannan MS, Prakash YS, Johnson DE, Sieck GC. Nitric oxide inhibits calcium release from sarcoplasmic reticulum of porcine tracheal smooth muscle cells. The American journal of physiology. 1997;272:L1–7. doi: 10.1152/ajplung.1997.272.1.L1. [DOI] [PubMed] [Google Scholar]
- Lam S, Chan H, LeRiche JC, Chan-Yeung M, Salari H. Release of leukotrienes in patients with bronchial asthma. The Journal of allergy and clinical immunology. 1988;81:711–717. doi: 10.1016/0091-6749(88)91043-3. [DOI] [PubMed] [Google Scholar]
- Mattoli S, Soloperto M, Marini M, Fasoli A. Levels of endothelin in the bronchoalveolar lavage fluid of patients with symptomatic asthma and reversible airflow obstruction. The Journal of allergy and clinical immunology. 1991;88:376–384. doi: 10.1016/0091-6749(91)90100-3. [DOI] [PubMed] [Google Scholar]
- McParland BE, Tait RR, Pare PD, Seow CY. The role of airway smooth muscle during an attack of asthma simulated in vitro. American journal of respiratory cell and molecular biology. 2005;33:500–504. doi: 10.1165/rcmb.2005-0183OC. [DOI] [PubMed] [Google Scholar]
- Montano LM, Bazan-Perkins B. Resting calcium influx in airway smooth muscle. Canadian journal of physiology and pharmacology. 2005;83:717–723. doi: 10.1139/y05-063. [DOI] [PubMed] [Google Scholar]
- Murray JJ, Tonnel AB, Brash AR, Roberts LJ, 2nd, Gosset P, Workman R, Capron A, Oates JA. Release of prostaglandin D2 into human airways during acute antigen challenge. The New England journal of medicine. 1986;315:800–804. doi: 10.1056/NEJM198609253151304. [DOI] [PubMed] [Google Scholar]
- Nials AT, Coleman RA, Johnson M, Magnussen H, Rabe KF, Vardey CJ. Effects of beta-adrenoceptor agonists in human bronchial smooth muscle. British journal of pharmacology. 1993;110:1112–1116. doi: 10.1111/j.1476-5381.1993.tb13929.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Donnell CR, Castile RG, Mead J. Changes in flow-volume curve configuration with bronchoconstriction and bronchodilation. Journal of Applied Physiology. 1986;61:2243–2251. doi: 10.1152/jappl.1986.61.6.2243. [DOI] [PubMed] [Google Scholar]
- Opazo Saez AM, Seow CY, Pare PD. Peripheral airway smooth muscle mechanics in obstructive airways disease. American journal of respiratory and critical care medicine. 2000;161:910–917. doi: 10.1164/ajrccm.161.3.9903138. [DOI] [PubMed] [Google Scholar]
- Orehek J, Douglas JS, Bouhuys A. Contractile responses of the guinea-pig trachea in vitro: modification by prostaglandin synthesis-inhibiting drugs. The Journal of pharmacology and experimental therapeutics. 1975;194:554–564. [PubMed] [Google Scholar]
- Prakash YS, Kannan MS, Walseth TF, Sieck GC. Role of cyclic ADPribose in the regulation of [Ca2+]i in porcine tracheal smooth muscle. The American journal of physiology. 1998;274:C1653–1660. doi: 10.1152/ajpcell.1998.274.6.C1653. [DOI] [PubMed] [Google Scholar]
- Rabe KF, Dent G, Magnussen H. Hydrogen peroxide contracts human airways in vitro: role of epithelium. The American journal of physiology. 1995;269:L332–338. doi: 10.1152/ajplung.1995.269.3.L332. [DOI] [PubMed] [Google Scholar]
- Rabe KF, Tenor H, Dent G, Schudt C, Liebig S, Magnussen H. Phosphodiesterase isozymes modulating inherent tone in human airways: identification and characterization. American journal of physiology. 1993;264:L458–464. doi: 10.1152/ajplung.1993.264.5.L458. [DOI] [PubMed] [Google Scholar]
- Scichilone N, Pyrgos G, Kapsali T, Anderlind C, Brown R, Permutt S, Togias A. Airways hyperresponsiveness and the effects of lung inflation. International Archives of Allergy & Immunology. 2001;124:262–266. doi: 10.1159/000053728. [DOI] [PubMed] [Google Scholar]
- Sieck GC, White TA, Thompson MA, Pabelick CM, Wylam ME, Prakash YS. Regulation of store-operated Ca2+ entry by CD38 in human airway smooth muscle. American journal of physiology. 2008;294:L378–385. doi: 10.1152/ajplung.00394.2007. [DOI] [PubMed] [Google Scholar]
- Skloot G, Permutt S, Togias A. Airway hyperresponsiveness in asthma: a problem of limited smooth muscle relaxation with inspiration. Journal of Clinical Investigation. 1995;96:2393–2403. doi: 10.1172/JCI118296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sollmann T, Gilbert AJ. Microscopic observations of bronchiolar reactions. The Journal of pharmacology and experimental therapeutics. 1937;61:272–285. [Google Scholar]
- Taylor SM, Pare PD, Schellenberg RR. Cholinergic and nonadrenergic mechanisms in human and guinea pig airways. Journal of applied physiology: respiratory, environmental and exercise physiology. 1984;56:958–965. doi: 10.1152/jappl.1984.56.4.958. [DOI] [PubMed] [Google Scholar]
- Vazquez G, Wedel BJ, Trebak M, St John Bird G, Putney JW., Jr Expression level of the canonical transient receptor potential 3 (TRPC3) channel determines its mechanism of activation. The Journal of biological chemistry. 2003;278:21649–21654. doi: 10.1074/jbc.M302162200. [DOI] [PubMed] [Google Scholar]
- Wang J, Laurier LG, Sims SM, Preiksaitis HG. Enhanced capacitative calcium entry and TRPC channel gene expression in human LES smooth muscle. Am J Physiol Gastrointest Liver Physiol. 2003;284:G1074–1083. doi: 10.1152/ajpgi.00227.2002. [DOI] [PubMed] [Google Scholar]
- Watson N, Magnussen H, Rabe KF. Inherent tone of human bronchus: role of eicosanoids and the epithelium. British journal of pharmacology. 1997;121:1099–1104. doi: 10.1038/sj.bjp.0701244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wenzel SE, Westcott JY, Larsen GL. Bronchoalveolar lavage fluid mediator levels 5 minutes after allergen challenge in atopic subjects with asthma: relationship to the development of late asthmatic responses. The Journal of allergy and clinical immunology. 1991;87:540–548. doi: 10.1016/0091-6749(91)90013-e. [DOI] [PubMed] [Google Scholar]
- White J, Eiser NM. The role of histamine and its receptors in the pathogenesis of asthma. British journal of diseases of the chest. 1983;77:215–226. [PubMed] [Google Scholar]
- White TA, Kannan MS, Walseth TF. Intracellular calcium signaling through the cADPR pathway is agonist specific in porcine airway smooth muscle. Faseb J. 2003;17:482–484. doi: 10.1096/fj.02-0622fje. [DOI] [PubMed] [Google Scholar]
- White TA, Xue A, Chini EN, Thompson M, Sieck GC, Wylam ME. Role of transient receptor potential C3 in TNF-alpha-enhanced calcium influx in human airway myocytes. American journal of respiratory cell and molecular biology. 2006;35:243–251. doi: 10.1165/rcmb.2006-0003OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wylam ME, Samsel RW, Schumacker PT, Umans JG. Extracellular calcium and intrinsic tone in the human umbilical artery. The Journal of pharmacology and experimental therapeutics. 1993;266:1475–1481. [PubMed] [Google Scholar]
- Yamakage M, Chen X, Tsujiguchi N, Kamada Y, Namiki A. Different inhibitory effects of volatile anesthetics on T- and L-type voltage-dependent Ca2+ channels in porcine tracheal and bronchial smooth muscles. Anesthesiology. 2001;94:683–693. doi: 10.1097/00000542-200104000-00024. [DOI] [PubMed] [Google Scholar]
- Zhao W, Guenard H. Effect of nitric oxide on in vitro responsiveness of bovine bronchus and pulmonary vessels. Eur Respir J. 1995;8:755–761. [PubMed] [Google Scholar]


