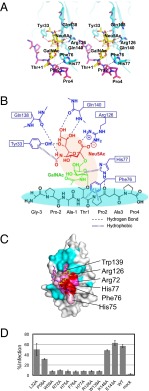
Fig. 4.
A detailed view of simultaneous recognition by PILRα of the O-glycan and peptide region of the sTn peptide and functional assays with the mutant PILRα proteins. (A) Stereoview of the interactions between PILRα and sTn peptide. (B) Schematic representation of the simultaneous recognition by PILRα of the O-glycan and the attached peptide of the sTn peptide. Sialic acid (red), GalNAc (green), the peptide region (black), and PILRα residues (blue) are shown. (C) gB binding with wild-type and mutant PILRα proteins. In the SPR study, the ligand was gB, and the analytes were the PILRα proteins. Significant residues that showed no (red) or reduced (pink) binding affinity based on the structure of the PILRα-sTn peptide complex are mapped. Cyan indicates residues in which mutations did not affect gB binding. (D) The bar graph shows the infectious activity of HSV-1 in cells expressing wild-type or mutant PILRα.