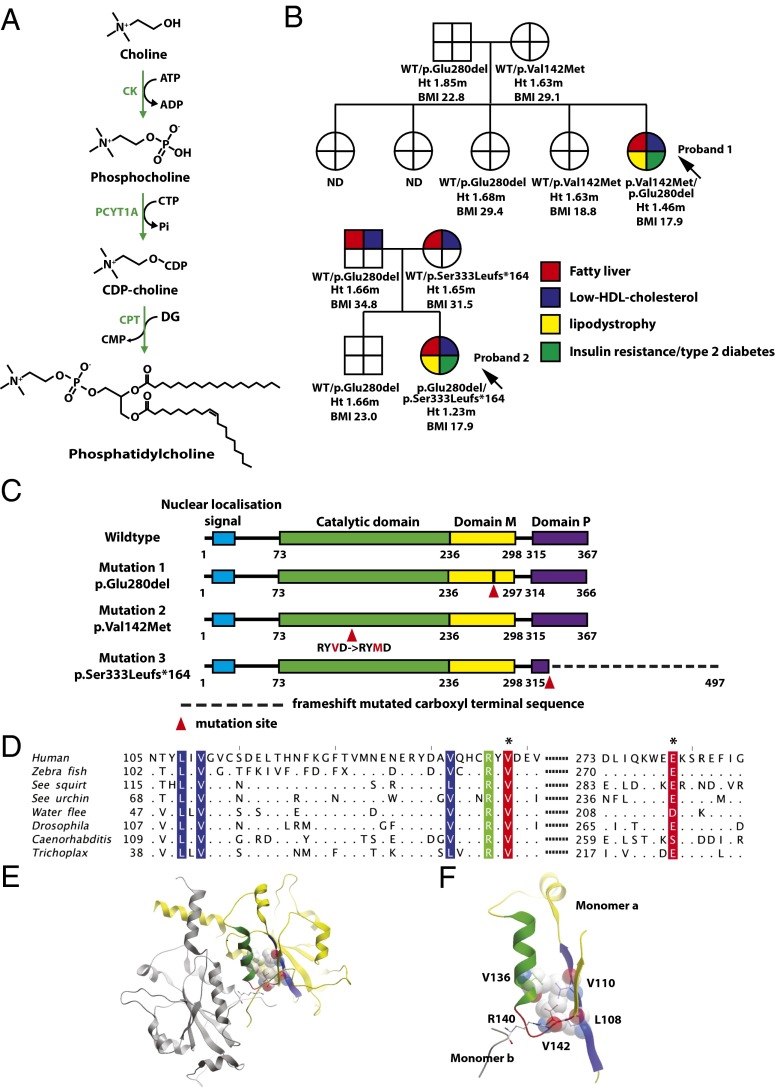
Fig. 1.
Cosegregation of biallelic PCYT1A mutations with fatty liver, low HDL cholesterol levels, lipodystrophy, insulin-resistant diabetes, and short stature. (A) Schematic illustration of the Kennedy PC synthesis pathway. CK, choline kinase; CPT, CDP-choline:1,2-diacylglycerol cholinephosphotransferase; PCYT1A, choline-phosphate cytidylyltransferase A, CTP:phosphocholine-cytidylyltransferase. (B) Family pedigrees of both probands demonstrating that only compound heterozygous carriers of PCYT1A mutations manifest fatty liver (red), low HDL cholesterol (blue), lipodystrophy (yellow), and insulin resistance/type 2 diabetes (T2DM) (green). PCYT1A mutation status, height (Ht.), and body mass index (BMI) are indicated below each individual’s symbol. ND, not determined; WT, wild type. (C) The location of PCYT1A mutations E280del, V142M, and 333fs in relation to known functional domains of PCYT1A. Domain M, membrane binding domain; domain P, phosphorylated region. (D) Conservation around the V142(red*) and E280(red*) mutation sites. Sequence alignment of representative metazoan sequences in the region surrounding the mutated residues. Hydrophobic (blue) and polar (green) residues interacting with V142 are highlighted. Only residues different from the human sequence are shown. Sequence IDs: human (Homo sapiens) P49585, zebrafish (Danio rerio) F1QEN6, sea squirt (Ciona intestinalis) XP_002130773.1, sea urchin (Strongylocentrotus purpuratus) H3I3V9, water flee (Daphnia pulex) E9G1P5, Drosophila (D. melanogaster) Q9W0D9, Caenorhabditis (C. elegans) P49583, Trichoplax (T. adherens) B3RI62. (E and F) Structure of the catalytic domain of PCYT1A highlighting the role of V142M in the core packing. The two chains in the dimer are shown in yellow and gray; the residues and the secondary structure units are highlighted in color in the yellow monomer A: loop L3 with V142, red; α-helix, green; and the interacting β-sheet, blue. The residues packing with V142 are shown in ball-and-stick and space-filling representations, the dimer stabilizing R140 is shown in ball-and-stick colored according to the atom type. E is a global view, and F is a zoomed-in view of the catalytic core.