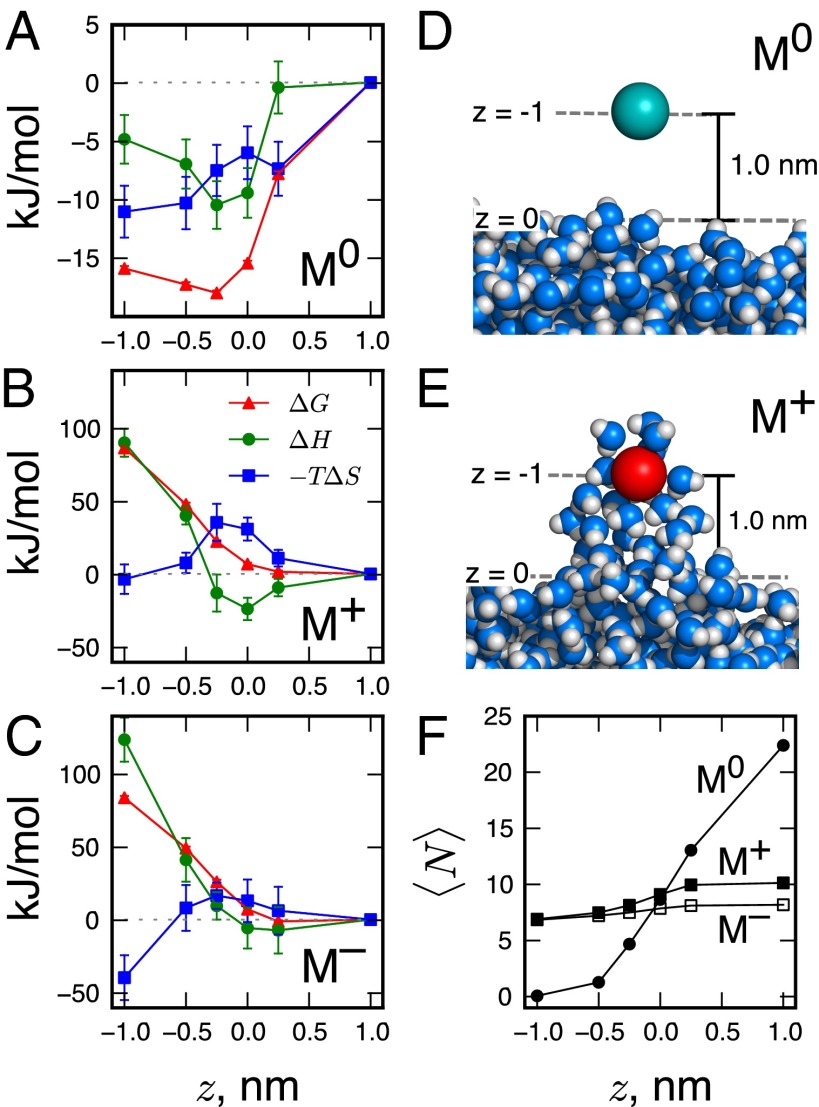
Fig. 3.
(A–C) Free energy , enthalpy , and entropy of moving a solute from bulk water to a given z location for (A) solute and (B) and (C) ions. (D and E) Simulation snapshots show that the solute is fully dehydrated (D), whereas the ion remains partially hydrated (E), deforming the interface when pulled to . (F) The average number, , of water oxygen centers in the first hydration shell of the ions and of the neutral solute at various z locations. The hydration shell radius is defined by the first minimum in the solute–water radial distribution function in bulk water.