Significance
Aphids are sap-feeding plant pests of great agricultural importance. Aphid saliva is known to modulate plant immune responses, but limited information exists about the composition of aphid saliva. By means of mass spectrometry, we identified 105 proteins in the saliva of the potato aphid Macrosiphum euphorbiae. Among these proteins were some originating from the proteobacterium Buchnera aphidicola, which lives endosymbiotically within bacteriocytes in the hemocoel of the aphid. We demonstrate that one of these endosymbiont-derived proteins, the chaperonin GroEL, is recognized by the plant immune surveillance system and activates pattern-triggered immunity. Our findings indicate that the outcome of plant–aphid interactions critically depends on a third element, the aphid endosymbiotic prokaryotic component, which induces plant immunity.
Keywords: salivary proteins, piercing-sucking insects
Abstract
Aphids are sap-feeding plant pests and harbor the endosymbiont Buchnera aphidicola, which is essential for their fecundity and survival. During plant penetration and feeding, aphids secrete saliva that contains proteins predicted to alter plant defenses and metabolism. Plants recognize microbe-associated molecular patterns and induce pattern-triggered immunity (PTI). No aphid-associated molecular pattern has yet been identified. By mass spectrometry, we identified in saliva from potato aphids (Macrosiphum euphorbiae) 105 proteins, some of which originated from Buchnera, including the chaperonin GroEL. Because GroEL is a widely conserved bacterial protein with an essential function, we tested its role in PTI. Applying or infiltrating GroEL onto Arabidopsis (Arabidopsis thaliana) leaves induced oxidative burst and expression of PTI early marker genes. These GroEL-induced defense responses required the known coreceptor BRASSINOSTEROID INSENSITIVE 1-ASSOCIATED RECEPTOR KINASE 1. In addition, in transgenic Arabidopsis plants, inducible expression of groEL activated PTI marker gene expression. Moreover, Arabidopsis plants expressing groEL displayed reduced fecundity of the green peach aphid (Myzus persicae), indicating enhanced resistance against aphids. Furthermore, delivery of GroEL into tomato (Solanum lycopersicum) or Arabidopsis through Pseudomonas fluorescens, engineered to express the type III secretion system, also reduced potato aphid and green peach aphid fecundity, respectively. Collectively our data indicate that GroEL is a molecular pattern that triggers PTI.
Plant sap-feeding insects such as aphids use a specialized elongated and flexible mouthpart, known as the stylets, to deliver saliva into the host and suck nutrients. During host penetration, aphids secrete two types of saliva: gelling saliva and watery saliva. The gelling saliva, which gels immediately upon deposition, forms a sheath around the stylets inside the plant tissue, and remains behind after the stylet is retracted. Recently, it has been shown that components of aphid saliva play a role in modulating plant host defense responses (1–3). Perception of microbial pathogenes by the host immune surveillance system is initiated by recognition of microbe-associated molecular patterns (MAMPs) by pattern recognition receptors (PRRs) whose activation results in pattern-triggered immunity (PTI) (4, 5). MAMPs are typically proteins or nucleic acids that are essential signature molecules of a class of microbes. It is not clear whether aphids induce PTI, and no aphid-associated molecular pattern(s) has yet been identified.
Aphids harbor Buchnera aphidicola, an obligate mutualist endosymbiotic γ-Protobacterium that has coevolved with the insect and is essential for its reproduction and survival (6). These bacteria are housed within bacteriocytes, specialized aphid cells in the insect hemocoel, where they function to provide essential amino acids (7, 8). A possible role for the Buchnera endosymbiont in plant–aphid interactions has been speculated (9), but no direct evidence for this interaction exists.
Recently, transcriptomic and proteomic analysis of pea aphid (Acyrthosiphum pisum) salivary glands identified 324 proteins, which based on the presence of secretion signal peptides are likely to be secreted (10). However, direct profiling of the aphid salivary proteome using mass spectrometry (MS) identified only about three dozen secreted proteins (11–16). This apparent discrepancy is likely due to the scarcity of saliva secreted by aphids in vitro.
To characterize the aphid salivary proteome, saliva was collected from a large number of potato aphids (Macrosiphum euphorbiae) and was profiled using high-throughput proteomics-based liquid chromatography, nanoelectrospray ionization, and tandem MS. Here we report the identification of aphid salivary proteins among which are proteins of the endosymbiont Buchnera origin and the recognition of one of these endosymbiont proteins, the chaperonin GroEL, by the plant innate immune system.
Results and Discussion
Aphid Saliva Contains Large Numbers of Proteins.
To characterize the aphid salivary proteome, liquid and gelling saliva were collected in vitro in water from about 100,000 potato aphids. Proteins in the two types of saliva were profiled using MS. To identify salivary proteins of aphid origin, the MS spectra were searched against a predicted potato aphid proteome, which was derived from transcriptome data, as the genome of the potato aphid has not been sequenced. We identified a total of 94 aphid proteins in combined gelling and liquid saliva (Dataset S1 and SI Appendix, Text). The great majority of these proteins were also predicted to be present in the genome of pea aphid whose genome sequence has been published (17). Only four of the identified 94 aphid proteins seem specific to potato aphids, which is likely an underestimate because of the incomplete nature of the potato aphid transcriptome (Dataset S1). Of these aphid proteins, seven (7.4%) were present only in the gelling saliva, whereas 44 (46.8%) were present in both liquid and gelling saliva, suggesting either these proteins have affinity to stick to the gelling matrix or remnants of the liquid saliva on the parafilm pouches could have cross-contaminated the gelling saliva. About 62 (66%) of these aphid proteins have no known function; the remaining ones represented proteins with a plethora of functions such as oxidative stress responses or alcohol, carbohydrate, and lipid metabolism (Dataset S1). A number of the aphid salivary proteins seem to be aphid-specific and therefore are excellent candidates for the development of durable pest-resistant crops using RNAi technology. Expressing silencing transcripts specifically targeting such aphid-specific genes in crops may result in high levels of pest resistance without leading to off-target effects.
Because not all of the potato aphid transcriptome sequences are full-length, we used the predicted proteins of their pea aphid orthologs to predict their secretion. About 67% of these aphid proteins were predicted to be secreted (Dataset S1). Among the salivary proteins not predicted for secretion were chaperonins, as well as proteins involved in energy metabolism or membrane trafficking, and components of the cytoskeleton. Saliva was collected within a period of 16 h. The absence of dead aphids during this period eliminated the possibility that these proteins were products of histolysis. To identify the source of these unexpected proteins, saliva collections were stained with 4,6'-diamidino-2-phenylindole (DAPI) to search for nuclei. No nuclei were detected in these collections (SI Appendix, Fig. S1).
Aphid Saliva Contains Proteins from the Endosymbiont Buchnera.
To identify proteins of endosymbiont origin in the potato aphid saliva, the obtained spectra were also searched against Buchnera-predicted proteins and 11 proteins were identified, four of which were detected only in the gelling saliva. Interestingly, among the Buchnera proteins was the chaperonin GroEL (Dataset S1). Of the 12 GroEL-matching peptides, five were specific to GroEL from Buchnera (Dataset S1). Using antibodies against Escherichia coli GroEL, the presence of this type of protein had been reported in aphid saliva (18). More recently, using proteomics, a single peptide matching to both E. coli and Buchnera GoEL has also been identified in aphid saliva (12). Because of the cross-reactivity of the GroEL antibody and the cross-match of the GroEL peptide to E. coli and Buchnera, a conclusive determination of the origin of the GroEL in aphid saliva could not be made.
Consistent with our finding of Buchnera proteins in the aphid saliva is the recent identification of GroEL and additional Buchnera proteins in the aphid honeydew collected while aphids were feeding on the host plant (19). Honeydew is the excreted sugary substance composed of ingested plant-derived and aphid-produced components. Because aphids ingest saliva while feeding (20) (SI Appendix, Text), honeydew is expected to contain salivary components.
All Buchnera proteins identified in the aphid saliva are abundant proteins, GroEL being the most abundant, constituting 10% of the Buchnera proteins (21). Because Buchnera are housed within bacteriocytes, the discovery of Buchnera proteins in the aphid saliva suggests that these proteins are present in the hemolymph and are likely released into the salivary duct by salivary gland cells. This release is likely to occur during bacteriocyte turnover and/or degeneration in the postreproductive aphid stage (22, 23). Because bacteriocytes contain a full set of eukaryotic organelles (24), it is likely that proteins of aphid origin in the saliva, not predicted for secretions, originate from these cells too. These aphid proteins presumably are also released into the hemocoel during bacteriocyte degeneration and move through the salivary gland cells. Movement of macromolecules from the hemocoel to the salivary gland is likely to be a common means for elimination of macromolecules in aphids (25–27) (SI Appendix, Text). How this movement is facilitated remains to be investigated.
GroEL Induces Enhanced Resistance to Aphids.
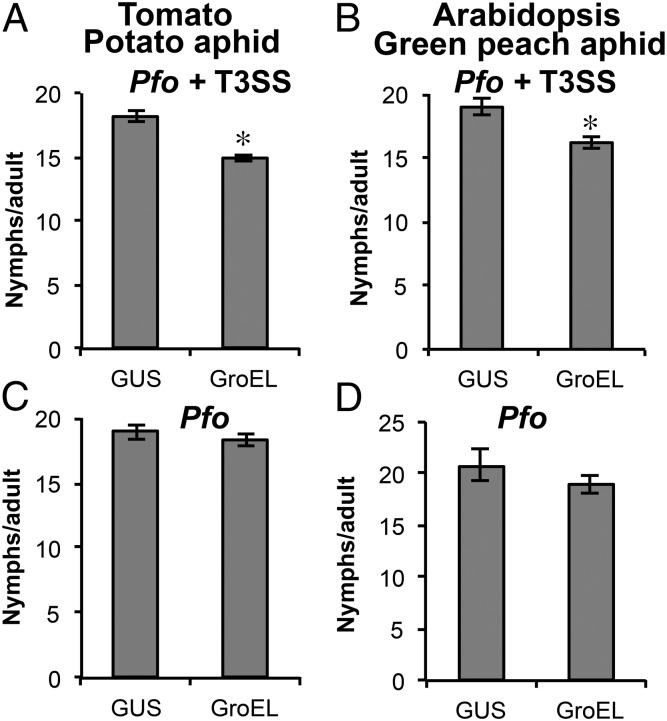
GroEL is one of the abundant proteins in bacteria and has been shown to elicit immune responses in animal systems (28). Because a great majority of aphids harbor Buchnera symbionts, we queried whether the Buchnera GroEL is recognized by the plant innate immunity and could serve as an aphid MAMP. To explore this possibility, we cloned Buchnera groEL from potato aphids (SI Appendix, Fig. S2) into the bacterial expression vector pVSP-PsSPdes, designed for delivery of effectors into plant cells through the type III secretion system (T3SS). We introduced this construct into an engineered Pseudomonas fluorescens, a nonpathogenic bacterium, with T3SS (Pfo+T3SS) for plant cell delivery (SI Appendix, Fig. S3). Unlike strains carrying the β-glucuronidase (GUS) control, tomato (Solanum lycopersicum) plants infected with Pfo carrying GroEL exhibited induction of PTI marker genes (SI Appendix, Fig. S4) and reduced aphid fecundity (Fig. 1A). These results indicate that the plant immune surveillance system recognized GroEL and triggered defense responses.
Fig. 1.
Delivery of GroEL into tomato and A. thaliana negatively affects aphid fecundity. Tomato and Arabidopsis were infiltrated with Pfo+T3SS or wild-type Pfo, carrying either GUS (control) or groEL, at a density of 1 × 104 cfu⋅ml−1. Plants were assayed with parthenogenetic potato aphids (A and C) or green peach aphids (B and D) and aphid fecundity was recorded daily over a 5-d period. Error bars represent ±SEM (in A and C, n = 18; B and D, n = 45). * indicates significant differences (Student t test; P < 0.05).
It is possible that GroEL could be present in the saliva of all aphids harboring Buchnera and therefore recognized by a multitude of plant hosts. Thus, we speculated that this induced host resistance might also be seen in other plant–aphid combinations. Because Buchnera GroEL sequences from different aphid species are highly conserved (SI Appendix, Fig. S2), we infected Arabidopsis thaliana (Arabidopsis) with Pfo+T3SS carrying Buchnera GroEL from potato aphid and assayed its effects on green peach aphid (Myzus persicae) infestation. Green peach aphid fecundity was reduced on Arabidopsis infected with Pfo+T3SS carrying GroEL, suggesting reduced susceptibility to aphids in general (Fig. 1B). To confirm that delivery of GroEL into the plant caused the reduced susceptibility in both hosts, we expressed GroEL in wild-type Pfo that lacks the T3SS and infected both tomato and Arabidopsis with their respective aphid pests. In neither of these host-pest systems was an effect on aphid fecundity observed (Fig. 1 C and D), indicating that GroEL is the cause of the reduced susceptibility to both aphid pests.
Transgenic Expression of GroEL Also Results in Enhanced Resistance to Aphids.
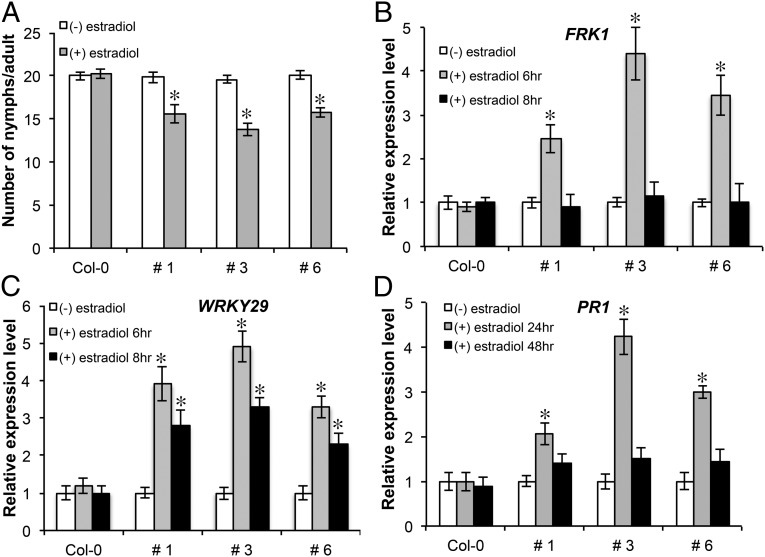
To substantiate the role of GroEL in immunity and aphid resistance, we developed transgenic Arabidopsis lines that overexpressed groEL constitutively or from an estradiol-inducible promoter (SI Appendix, Fig. S5 A and B). In agreement with our earlier finding, transgenic expression of groEL in Arabidopsis reduced aphid fecundity (Fig. 2A and SI Appendix, Fig. S5D). A previous study found that leaf infiltration of an aphid salivary fraction that should have contained GroEL did not reduce aphid fecundity (3). This apparent discrepancy might be explained by the presence of other proteins that counteract GroEL-induced defenses. Future studies might reveal the identity of these putative effectors with the ability to suppress GroEL-induced plant defense. Besides the presence of GroEL-induced PTI suppressors in this fraction, the lengthier period (24 h compared with 16 h) dedicated for collection of aphid saliva and its fractionation on columns might have caused degradation of the proteins in this selected size range.
Fig. 2.
A. thaliana Col-0 transgenic lines expressing GroEL exhibit enhanced resistance to aphids. (A) Fecundity of green peach aphids on Arabidopsis transgenic lines (1, 3, and 6) expressing β-estradiol–inducible GroEL, recorded daily over a 5-d period. Error bars represent ±SEM (n = 30). GroEL induces defense marker gene expression. FRK1 (B), WRKY29 (C), and PR1 (D) expression were evaluated in the three Arabidopsis transgenic lines. In B–D, error bars represent ±SEM of six biological replicates and two technical replicates each. * indicates significant differences from uninduced (–) estradiol control (Student t test; P < 0.05).
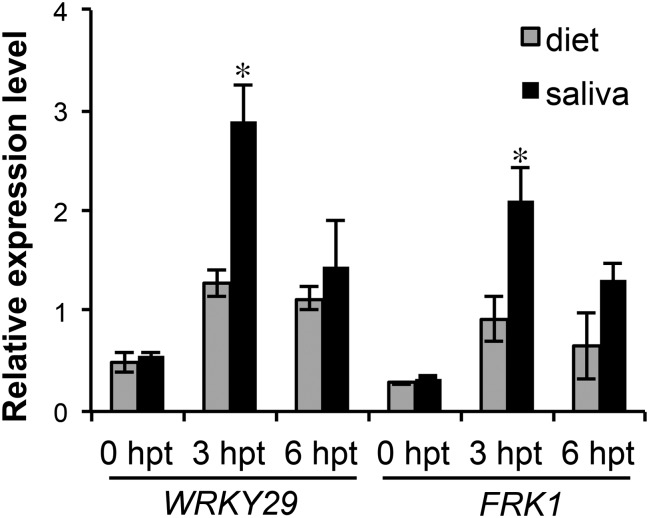
Transgenic Arabidopsis lines expressing GroEL induced expression of both early [FLG22-INDUCED RECEPTOR-LIKE KINASE 1 (FRK1) and transcription factor WRKY29; Fig. 2 B and C] and late [PATHOGENESIS RELATED 1 (PR1); Fig. 2D and SI Appendix, Fig. S5C] PTI marker genes. PR1 is known to be induced by aphid feeding (29, 30), but no information was available on FRK1 and WRKY29 expression in early infestation stages (3, 31, 32). Hence, to assess their expression during aphid defense, we infiltrated green peach aphid saliva into Arabidopsis leaves. Expression of both FRK1 and WRKY29 were induced early and transiently (Fig. 3), indicating a possible role for these genes in aphid defense.
Fig. 3.
Aphid saliva induces early-induced defense marker genes in Arabidopsis. Arabidopsis plants were infiltrated with diet only and diet fed on green peach aphids (saliva). Leaf samples were harvested at 0 h posttreatment (hpt), 3 hpt, and 6 hpt. Relative expression levels of defense marker genes were evaluated by quantitative RT-PCR. Error bars represent ±SEM of six biological replicates and two technical replicates each. * indicates significant differences from diet-only control (Student t test; P < 0.05).
Contrary to endosymbiont GroEL inducing defense against aphids, symbiotic bacteria present in oral secretions of chewing insect larvae have been shown to modulate plant defenses to enhance larval performance (33). For example, the Colorado beetle larvae exploit the antagonistic relationship between the plant defense hormones salicylic acid (SA) and jasmonic acid (JA) to manipulate plant defense to their advantage (33). Bacteria in beetle larval oral secretions activate SA-regulated responses to suppress JA-regulated responses that are effective against these larvae. Thus, beetle larvae manipulate host defenses through their bacterial symbiont for their own advantage.
GroEL Treatment Induces PTI.
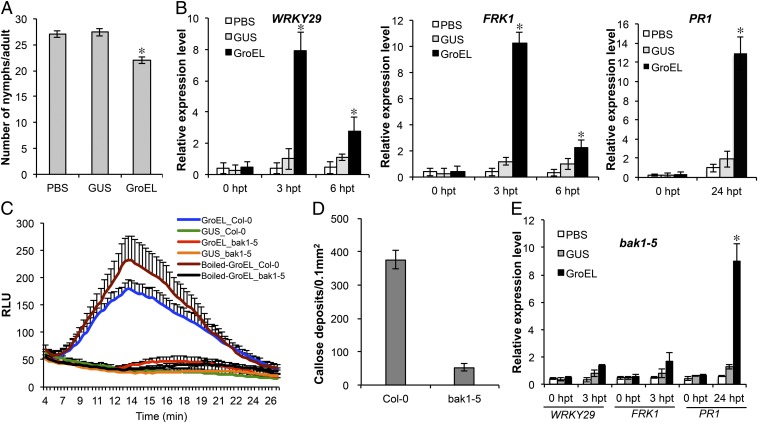
To further characterize GroEL-induced PTI, we expressed histidine (His) epitope-tagged GroEL in E. coli and purified the recombinant protein using nickel-NTA beads followed by anion exchange chromatography (SI Appendix, Fig. S6). We infiltrated Arabidopsis leaves with the purified GroEL and assayed for PTI responses. His-tagged and purified GUS was used as control. To test whether extracellular application of GroEL induces enhanced aphid resistance, GroEL-infiltrated plants were first assayed for aphid fecundity. Plants treated with GroEL did not exhibit a visible immune response such as cell death. However, they displayed reduced aphid fecundity (Fig. 4A) similar to Pfo delivery or transgenic expression of GroEL. Similarly, infiltration of GroEL into leaves induced expression of both early- and late-induced PTI marker genes (Fig. 4B). In addition, GroEL triggered reactive oxygen species (ROS) accumulation (Fig. 4C) and callose deposition in treated leaves (Fig. 4D and SI Appendix, Fig. S7A). None of these defense responses were detected in the GUS-treated control leaves (Fig. 4). To assess whether GroEL chaperonin activity is required for defense induction, GroEL was boiled and used immediately in the ROS assay. Boiled GroEL triggered strong ROS activity (Fig. 4C), indicating that the molecular pattern of the denatured GroEL is the defense trigger. Taken together, these results indicate that GroEL serves as a microbe/aphid-associated molecular signature that induces PTI.
Fig. 4.
GroEL-induced defense responses in Arabidopsis is BAK1-dependent. (A) Green peach aphid fecundity on Col-0 plants infiltrated with PBS (buffer), 1.5 μM GUS (control), or GroEL recorded over a 5 d period. Error bars represent ±SEM (n = 30). * indicates significant differences (ANOVA Tukey HSD test; P < 0.05). (B) Expression of defense marker genes in Col-0 leaves infiltrated with PBS or 1.5 μM GroEL or GUS at the indicated hpt. Error bars represent ±SEM of six biological replicates and two technical replicates. (C) Oxidative burst triggered by 1.5 μM GroEL, boiled GroEL or GUS in Col-0, and bak1-5 leaves measured in relative luminescence units (RLUs). Error bars represent ± SEM (n = 15). (D) Callose deposition in Col-0 and bak1-5 leaves infiltrated with 1.5 μM GroEL. Error bars represent ±SEM (n = 16). (E) Expression of defense marker genes in bak1-5 leaves performed as described for B. For B and E, * indicates significant differences for each gene at a time point (ANOVA Tukey HSD test; P < 0.05).
Because most bacterial PTI responses require the well-characterized BRI-ASSOCIATED RECEPTOR KINASE 1 (BAK1) coreceptor (34), we tested whether GroEL-induced ROS and callose deposition are also BAK1-dependent. We used the bak1-5 mutant that has a substitution in the cytoplasmic kinase domain leading to compromised innate immune signaling (35). The ROS burst (Fig. 4C) and callose deposition (Fig. 4D and SI Appendix, Fig. S7B) triggered by GroEL were both greatly reduced in the bak1-5 mutant, indicating BAK1 dependency. Similarly, GroEL-induced expression of the PTI early-induced marker genes (WRKY29 and FRK1), which were known to be BAK1-dependent (36, 37), and not the late-induced marker gene (PR1) (38), were impaired in bak1-5 (Fig. 4E).
Arabidopsis is a nonhost to the pea aphid, which does not feed on this plant or other brassicaceae, whereas green peach aphid can use Arabidopsis as a host. It was recently shown that pea aphids survived longer on the bak1-5 mutant compared with Arabidopsis Col-0, whereas no effect on green peach aphid survival was detected on bak1-5 plants. This shows that BAK1 contributes to nonhost resistance to aphids (39). BAK1 could be participating in PTI triggered by recognition of a number aphid-associated molecular patterns including GroEL. Considering that application of GroEL reduced green peach aphid fecundity whereas no enhanced survival was reported to this aphid on bak1-5 (39), our results suggest that this Arabidopsis-adapted aphid has evolved effectors that are able to suppress BAK1-dependent PTI. This is in agreement with a previous report where a green peach aphid effector was shown to suppress PTI (2). Alternatively, because not all GroEL-induced PTI responses were impaired in the bak1-5 mutant, GroEL may partially promote resistance against green peach aphid in a BAK1-independent manner.
To conclude, aphid saliva contains proteins of both aphid and endosymbiont Buchnera origins. The presence of Buchnera proteins in the potato aphid saliva indicates a major role for this endosymbiont in aphid–plant interactions. Although the ability of GroEL to elicit plant PTI is not surprising, it is interesting that this defense is effective against aphids. It is intriguing to speculate that plant defenses directly target the Buchnera endosymbiont to control the insect pest. Because the aphid–Buchnera mutualism is obligate, where none of the partners can survive without the other, by targeting the endosymbiont the plant immune system is exploiting the strict mutual dependency of a host-insect with its symbiont to recognize the former as the intruder.
Our study is based exclusively on in planta overexpression of GroEL or exogenous application of the purified protein. Direct assessment of the in vivo significance of GroEL in aphid–plant interactions is not feasible, because elimination of this critical chaperone from Buchnera is likely lethal, and successful genetic manipulation of this endosymbiont has not been reported.
Our data further suggest that GroEL is recognized both extracellularly and intracellularly (in transgenic groEL-expressing plants), which may reflect aphid salivation behavior during probing and feeding. Although aphid salivation occurs mainly in the sieve element, watery saliva is injected frequently into the plant apoplast and nonvascular cells during brief intracellular punctures by the stylets during plant penetration phase (20). Alternatively, GroEL may be leaking outside the cell in the transgenic groEL-overexpressing plants (40) and solely be recognized extracellularly through a transmembrane receptor similar to well-characterized microbial MAMPs (41). How GroEL is recognized by the plant innate immunity is not yet known. Although BAK1 is a transmembrane receptor, it is unlikely that BAK1 itself is the receptor for GroEL, but it likely acts as a coreceptor analogous to its function for the bacterial MAMP receptor FLS2 (42). Therefore, recognition of GroEL likely involves a yet unidentified receptor.
Materials and Methods
Plant Material and Growth Conditions.
Tomato cultivar Moneymaker plants were maintained as described previously (43). Arabidopsis bak1-5 mutant, in a Col-0 genetic background (44), and wild-type Arabidopsis Col-0 plants were grown under a 12 h light photoperiod. Unless mentioned otherwise, 5-wk-old tomato and Arabidopsis plants were used for assays.
Aphid Colonies and Growth Conditions.
Colonies of the parthenogenetic potato aphid (M. euphorbiae) and green peach aphid (M. persicae) were reared on tomato cv. UC82B and mustard India plants, respectively, and maintained as described in refs. 43, 45. Age-synchronized 1-d-old adult aphids were produced as described in ref. 46.
Saliva Collection from Potato Aphids.
To collect saliva, potato aphids were fed on ultra pure sterile water in parafilm pouches as described previously (47). Saliva was collected from an estimated 100,000 aphids. Additional details are in SI Appendix, Materials and Methods.
Saliva Preparation and Liquid Chromatography–MS Analysis.
MS analysis was performed as described previously (48). Details are in SI Appendix, Materials and Methods.
Annotation, Gene Ontology Classification, and Signal Peptide Prediction.
Potato aphid transcripts, matching to the sequenced peptides, were annotated, and amino acid sequences of their putative full-length pea aphid orthologs were subjected to de novo signal peptide prediction analysis using SignalP 4.0 (49) and TargetP 1.1 (50). Additional details are in SI Appendix, Materials and Methods.
DAPI Staining.
Aphid ovaries and saliva were fixed in 1% paraformaldehyde, and nuclei were stained with 1 µg/mL of DAPI (Sigma). Samples were observed under a fluorescence microscope (Nikon Eclipse Ti). Additional details are in SI Appendix, Materials and Methods.
Cloning in pVSP PsSPdes Vector and Aphid Bioassays.
groEL (accession no. KF366417) and GUS were PCR amplified from M. euphorbiae gDNA and pENTR-GUS (Invitrogen), respectively. Products were cloned into the pVSP PsSPdes vector (51) as described previously (45) and transformed into an engineered Pfo strain (EtHAn) with T3SS (52) and wild-type Pfo. Details are in SI Appendix, Materials and Methods.
Leaves of 5-wk-old Arabidopsis plants were infiltrated with Pfo, and 24 h later, plants were infested with a single age-synchronized 1-d-old adult green peach aphid. Tomato assays were performed as described previously (45). Plants were infested with nine age-synchronized 1-d-old adult potato aphids 24 h after infiltration. Aphid fecundity was assessed by counting the number of nymphs daily for a period of 5 d. Details of plant treatment and aphid infestation are in SI Appendix, Materials and Methods.
Construction of Transgenic Plants Expressing GroEL.
Arabidopsis Col-0 plants were used to generate GroEL transgenic lines using Agrobacterium tumefaciens-mediated floral-dip transformation (53). Details are in SI Appendix, Materials and Methods.
Aphid Bioassays on Transgenic Arabidopsis.
Five-week-old transgenic plants were sprayed with 20 µM β-estradiol solution, and 24 h later, plants were infested with aphids. Details of aphid infestation are in SI Appendix, Materials and Methods.
Expression and Purification of Proteins.
GroEL and GUS His-fusion proteins were developed as described in SI Appendix, Materials and Methods. Proteins were expressed and purified using a nickel-NTA column (QIAGEN) as described previously (54). Eluted GroEL protein was further fractionated using anion exchange chromatography by AthenaES (Athena Enzyme Systems Group).
Aphid Bioassays with Purified GroEL Protein.
Arabidopsis leaves were infiltrated with GroEL using a 1 mL needle-less syringe and used in aphid assays. Details are in SI Appendix, Materials and Methods.
Saliva Collection from Green Peach Aphid and Arabidopsis Treatment.
Green peach aphid saliva was collected in a diet containing sucrose and amino acids as described in ref. 55. Diet was infiltrated into Arabidopsis leaves and harvested immediately after infiltration at 0 h and at 3 h and 6 h posttreatment. Details are in SI Appendix, Materials and Methods.
Quantitative Real-Time PCR Analysis.
RNA extraction and sample preparation for quantitative RT-PCR was performed as described earlier (43) using gene-specific primers (SI Appendix, Table S1). Relative expression of genes was calculated using actin (ACT-2) as a standard gene for Arabidopsis and ubiquitin (Ubi3) for tomato (SI Appendix, Table S1). Details for plant growth conditions and treatments are in SI Appendix, Materials and Methods.
Oxidative Burst and Callose Deposition.
The ROS burst was determined by a luminol-based assay as described previously (56). Callose deposition was performed as described previously (57). Details are in SI Appendix, Materials and Methods.
Supplementary Material
Acknowledgments
We thank F. Takken (University of Amsterdam), A. Dahanukar, and T. Eulgem (both University of California, Riverside) for comments on the manuscript; G. Walker, C. Weirauch, L. Walling, J. Ng, and H. Jin (all University of California, Riverside) for discussions; and Cyril Zipfel (The Sainsbury Laboratory) for Arabdopsis bak1-5 mutant. This work was supported by funding from US Department of Agriculture-National Institute of Food and Agriculture Award 2010-65106-20675 (to I.K.) and National Science Foundation Award 0619411 (to S.P.B.).
Footnotes
The authors declare no conflict of interest.
Data deposition: The sequences reported in this paper have been deposited in the GenBank database [SRX339176 (potato aphid transcriptome), GAOM00000000 (transcripts encoding potato aphid salivary proteins), and KF366417 (Buchnera groEL from potato aphids)].
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1407687111/-/DCSupplemental.
References
- 1.Will T, Tjallingii WF, Thönnessen A, van Bel AJ. Molecular sabotage of plant defense by aphid saliva. Proc Natl Acad Sci USA. 2007;104(25):10536–10541. doi: 10.1073/pnas.0703535104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bos JI, et al. A functional genomics approach identifies candidate effectors from the aphid species Myzus persicae (green peach aphid) PLoS Genet. 2010;6(11):e1001216. doi: 10.1371/journal.pgen.1001216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.De Vos M, Jander G. Myzus persicae (green peach aphid) salivary components induce defence responses in Arabidopsis thaliana. Plant Cell Environ. 2009;32(11):1548–1560. doi: 10.1111/j.1365-3040.2009.02019.x. [DOI] [PubMed] [Google Scholar]
- 4.Jones JD, Dangl JL. The plant immune system. Nature. 2006;444(7117):323–329. doi: 10.1038/nature05286. [DOI] [PubMed] [Google Scholar]
- 5.Ronald PC, Beutler B. Plant and animal sensors of conserved microbial signatures. Science. 2010;330(6007):1061–1064. doi: 10.1126/science.1189468. [DOI] [PubMed] [Google Scholar]
- 6.Wilson AC, et al. Genomic insight into the amino acid relations of the pea aphid, Acyrthosiphon pisum, with its symbiotic bacterium Buchnera aphidicola. Insect Mol Biol. 2010;19(Suppl 2):249–258. doi: 10.1111/j.1365-2583.2009.00942.x. [DOI] [PubMed] [Google Scholar]
- 7.Moran NA, Munson MA, Baumann P, Ishikawa H. A molecular clock in endosymbiotic bacteria is calibrated using the insect hosts. Proc Biol Sci. 1993;253(1337):167–171. [Google Scholar]
- 8.Akman Gündüz E, Douglas AE. Symbiotic bacteria enable insect to use a nutritionally inadequate diet. Proc Biol Sci. 2009;276(1658):987–991. doi: 10.1098/rspb.2008.1476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kaloshian I. Gene-for-gene disease resistance: Bridging insect pest and pathogen defense. J Chem Ecol. 2004;30(12):2419–2438. doi: 10.1007/s10886-004-7943-1. [DOI] [PubMed] [Google Scholar]
- 10.Carolan JC, et al. Predicted effector molecules in the salivary secretome of the pea aphid (Acyrthosiphon pisum): A dual transcriptomic/proteomic approach. J Proteome Res. 2011;10(4):1505–1518. doi: 10.1021/pr100881q. [DOI] [PubMed] [Google Scholar]
- 11.Cooper WR, Dillwith JW, Puterka GJ. Salivary proteins of Russian wheat aphid (Hemiptera: Aphididae) Environ Entomol. 2010;39(1):223–231. doi: 10.1603/EN09079. [DOI] [PubMed] [Google Scholar]
- 12.Vandermoten S, et al. Comparative analyses of salivary proteins from three aphid species. Insect Mol Biol. 2014;23(1):67–77. doi: 10.1111/imb.12061. [DOI] [PubMed] [Google Scholar]
- 13.Rao SA, Carolan JC, Wilkinson TL. Proteomic profiling of cereal aphid saliva reveals both ubiquitous and adaptive secreted proteins. PLoS ONE. 2013;8(2):e57413. doi: 10.1371/journal.pone.0057413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nicholson SJ, Hartson SD, Puterka GJ. Proteomic analysis of secreted saliva from Russian Wheat Aphid (Diuraphis noxia Kurd.) biotypes that differ in virulence to wheat. J Proteomics. 2012;75(7):2252–2268. doi: 10.1016/j.jprot.2012.01.031. [DOI] [PubMed] [Google Scholar]
- 15.Carolan JC, Fitzroy CI, Ashton PD, Douglas AE, Wilkinson TL. The secreted salivary proteome of the pea aphid Acyrthosiphon pisum characterised by mass spectrometry. Proteomics. 2009;9(9):2457–2467. doi: 10.1002/pmic.200800692. [DOI] [PubMed] [Google Scholar]
- 16.Harmel N, et al. Identification of aphid salivary proteins: A proteomic investigation of Myzus persicae. Insect Mol Biol. 2008;17(2):165–174. doi: 10.1111/j.1365-2583.2008.00790.x. [DOI] [PubMed] [Google Scholar]
- 17.International Aphid Genomics Consortium Genome sequence of the pea aphid Acyrthosiphon pisum. PLoS Biol. 2010;8(2):e1000313. doi: 10.1371/journal.pbio.1000313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Filichkin SA, Brumfield S, Filichkin TP, Young MJ. In vitro interactions of the aphid endosymbiotic SymL chaperonin with barley yellow dwarf virus. J Virol. 1997;71(1):569–577. doi: 10.1128/jvi.71.1.569-577.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sabri A, et al. Proteomic investigation of aphid honeydew reveals an unexpected diversity of proteins. PLoS ONE. 2013;8(9):e74656. doi: 10.1371/journal.pone.0074656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tjallingii WF. Salivary secretions by aphids interacting with proteins of phloem wound responses. J Exp Bot. 2006;57(4):739–745. doi: 10.1093/jxb/erj088. [DOI] [PubMed] [Google Scholar]
- 21.Baumann P, Baumann L, Clark MA. Levels of Buchnera aphidicola chaperonin groEL during growth of the aphid Schizaphis graminum. Curr Microbiol. 1996;32(5):279–285. [Google Scholar]
- 22.Nishikori K, Morioka K, Kubo T, Morioka M. Age- and morph-dependent activation of the lysosomal system and Buchnera degradation in aphid endosymbiosis. J Insect Physiol. 2009;55(4):351–357. doi: 10.1016/j.jinsphys.2009.01.001. [DOI] [PubMed] [Google Scholar]
- 23.Douglas AE, Dixon AFG. The mycetocyte symbiosis in aphids: Variation with age and morph in virginoparae of Megoura viciae and Acyrthosiphon pisum. J Insect Physiol. 1987;33(2):109–113. [Google Scholar]
- 24.Griffiths GW, Beck SD. Ultrastructure of pea aphid mycetocytes: Evidence for symbiote secretion. Cell Tissue Res. 1975;159(3):351–367. doi: 10.1007/BF00221782. [DOI] [PubMed] [Google Scholar]
- 25.Ponsen MB. The site of potato leafroll virus multiplication in its vector, Myzus persicae: An anatomical study. Meded Landbou Wagen. 1972;16:1–147. [Google Scholar]
- 26.Miles PW. Insect secretions in plants. Annu Rev Phytopathol. 1968;6:137–164. [Google Scholar]
- 27.Miles PW. The saliva of Hemiptera. Adv Insect Physiol. 1972;9:183–255. [Google Scholar]
- 28.Henderson B, Allan E, Coates AR. Stress wars: The direct role of host and bacterial molecular chaperones in bacterial infection. Infect Immun. 2006;74(7):3693–3706. doi: 10.1128/IAI.01882-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Martinez de Ilarduya O, Xie Q, Kaloshian I. Aphid-induced defense responses in Mi-1-mediated compatible and incompatible tomato interactions. Mol Plant Microbe Interact. 2003;16(8):699–708. doi: 10.1094/MPMI.2003.16.8.699. [DOI] [PubMed] [Google Scholar]
- 30.Moran PJ, Thompson GA. Molecular responses to aphid feeding in Arabidopsis in relation to plant defense pathways. Plant Physiol. 2001;125(2):1074–1085. doi: 10.1104/pp.125.2.1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Moran PJ, Cheng Y, Cassell JL, Thompson GA. Gene expression profiling of Arabidopsis thaliana in compatible plant-aphid interactions. Arch Insect Biochem Physiol. 2002;51(4):182–203. doi: 10.1002/arch.10064. [DOI] [PubMed] [Google Scholar]
- 32.De Vos M, et al. Signal signature and transcriptome changes of Arabidopsis during pathogen and insect attack. Mol Plant Microbe Interact. 2005;18(9):923–937. doi: 10.1094/MPMI-18-0923. [DOI] [PubMed] [Google Scholar]
- 33.Chung SH, et al. Herbivore exploits orally secreted bacteria to suppress plant defenses. Proc Natl Acad Sci USA. 2013;110(39):15728–15733. doi: 10.1073/pnas.1308867110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dou D, Zhou JM. Phytopathogen effectors subverting host immunity: Different foes, similar battleground. Cell Host Microbe. 2012;12(4):484–495. doi: 10.1016/j.chom.2012.09.003. [DOI] [PubMed] [Google Scholar]
- 35.Schwessinger B, et al. Phosphorylation-dependent differential regulation of plant growth, cell death, and innate immunity by the regulatory receptor-like kinase BAK1. PLoS Genet. 2011;7(4):e1002046. doi: 10.1371/journal.pgen.1002046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Belkhadir Y, et al. Brassinosteroids modulate the efficiency of plant immune responses to microbe-associated molecular patterns. Proc Natl Acad Sci USA. 2012;109(1):297–302. doi: 10.1073/pnas.1112840108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shan L, et al. Bacterial effectors target the common signaling partner BAK1 to disrupt multiple MAMP receptor-signaling complexes and impede plant immunity. Cell Host Microbe. 2008;4(1):17–27. doi: 10.1016/j.chom.2008.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Larroque M, et al. Pathogen-associated molecular pattern-triggered immunity and resistance to the root pathogen Phytophthora parasitica in Arabidopsis. J Exp Bot. 2013;64(12):3615–3625. doi: 10.1093/jxb/ert195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Prince DC, Drurey C, Zipfel C, Hogenhout S. The leucine-rich repeat receptor-like kinase BAK1 and the cytochrome P450 PAD3 contribute to innate immunity to aphids in Arabidopsis. Plant Physiol. 2014 doi: 10.1104/pp.114.235598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wei HL, Chakravarthy S, Worley JN, Collmer A. Consequences of flagellin export through the type III secretion system of Pseudomonas syringae reveal a major difference in the innate immune systems of mammals and the model plant Nicotiana benthamiana. Cell Microbiol. 2012;15(4):601–618. doi: 10.1111/cmi.12059. [DOI] [PubMed] [Google Scholar]
- 41.Segonzac C, Zipfel C. Activation of plant pattern-recognition receptors by bacteria. Curr Opin Microbiol. 2011;14(1):54–61. doi: 10.1016/j.mib.2010.12.005. [DOI] [PubMed] [Google Scholar]
- 42.Sun Y, et al. Structural basis for flg22-induced activation of the Arabidopsis FLS2-BAK1 immune complex. Science. 2013;342(6158):624–628. doi: 10.1126/science.1243825. [DOI] [PubMed] [Google Scholar]
- 43.Bhattarai KK, Atamian HS, Kaloshian I, Eulgem T. WRKY72-type transcription factors contribute to basal immunity in tomato and Arabidopsis as well as gene-for-gene resistance mediated by the tomato R gene Mi-1. Plant J. 2010;63(2):229–240. doi: 10.1111/j.1365-313X.2010.04232.x. [DOI] [PubMed] [Google Scholar]
- 44.Roux M, et al. The Arabidopsis leucine-rich repeat receptor-like kinases BAK1/SERK3 and BKK1/SERK4 are required for innate immunity to hemibiotrophic and biotrophic pathogens. Plant Cell. 2011;23(6):2440–2455. doi: 10.1105/tpc.111.084301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Atamian HS, et al. In planta expression or delivery of potato aphid Macrosiphum euphorbiae effectors Me10 and Me23 enhances aphid fecundity. Mol Plant Microbe Interact. 2013;26(1):67–74. doi: 10.1094/MPMI-06-12-0144-FI. [DOI] [PubMed] [Google Scholar]
- 46.Bhattarai KK, Xie QG, Pourshalimi D, Younglove T, Kaloshian I. Coil-dependent signaling pathway is not required for Mi-1-mediated potato aphid resistance. Mol Plant Microbe Interact. 2007;20(3):276–282. doi: 10.1094/MPMI-20-3-0276. [DOI] [PubMed] [Google Scholar]
- 47.Miles PW. Studies on the salivary physiology of plant-bugs: The salivary secretions of aphids. J Insect Physiol. 1965;11(9):1261–1268. doi: 10.1016/0022-1910(65)90119-8. [DOI] [PubMed] [Google Scholar]
- 48.Bellafiore S, et al. Direct identification of the Meloidogyne incognita secretome reveals proteins with host cell reprogramming potential. PLoS Pathog. 2008;4(10):e1000192. doi: 10.1371/journal.ppat.1000192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Petersen TN, Brunak S, von Heijne G, Nielsen H. SignalP 4.0: Discriminating signal peptides from transmembrane regions. Nat Methods. 2011;8(10):785–786. doi: 10.1038/nmeth.1701. [DOI] [PubMed] [Google Scholar]
- 50.Emanuelsson O, Nielsen H, Brunak S, von Heijne G. Predicting subcellular localization of proteins based on their N-terminal amino acid sequence. J Mol Biol. 2000;300(4):1005–1016. doi: 10.1006/jmbi.2000.3903. [DOI] [PubMed] [Google Scholar]
- 51.Rentel MC, Leonelli L, Dahlbeck D, Zhao B, Staskawicz BJ. Recognition of the Hyaloperonospora parasitica effector ATR13 triggers resistance against oomycete, bacterial, and viral pathogens. Proc Natl Acad Sci USA. 2008;105(3):1091–1096. doi: 10.1073/pnas.0711215105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Thomas WJ, Thireault CA, Kimbrel JA, Chang JH. Recombineering and stable integration of the Pseudomonas syringae pv. syringae 61 hrp/hrc cluster into the genome of the soil bacterium Pseudomonas fluorescens Pf0-1. Plant J. 2009;60(5):919–928. doi: 10.1111/j.1365-313X.2009.03998.x. [DOI] [PubMed] [Google Scholar]
- 53.Clough SJ, Bent AF. Floral dip: A simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J. 1998;16(6):735–743. doi: 10.1046/j.1365-313x.1998.00343.x. [DOI] [PubMed] [Google Scholar]
- 54.Kale SD, et al. External lipid PI3P mediates entry of eukaryotic pathogen effectors into plant and animal host cells. Cell. 2010;142(2):284–295. doi: 10.1016/j.cell.2010.06.008. [DOI] [PubMed] [Google Scholar]
- 55.Kim JH, Jander G. Myzus persicae (green peach aphid) feeding on Arabidopsis induces the formation of a deterrent indole glucosinolate. Plant J. 2007;49(6):1008–1019. doi: 10.1111/j.1365-313X.2006.03019.x. [DOI] [PubMed] [Google Scholar]
- 56.Keppler LD, Baker CJ. O2-initiated lipid peroxidation in a bacteria-induced hypersensitive reaction in tobacco cell suspensions. Phytopathology. 1989;79(5):555–562. [Google Scholar]
- 57.Adam L, Somerville SC. Genetic characterization of five powdery mildew disease resistance loci in Arabidopsis thaliana. Plant J. 1996;9(3):341–356. doi: 10.1046/j.1365-313x.1996.09030341.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.