Abstract
Objective
To review the available evidence on the effectiveness of intrathecal baclofen in the treatment of spasticity in individuals with spinal cord injuries (SCIs) at least 6 months post-injury or diagnosis.
Data sources
A literature search of multiple databases (Pub Med, CINAHL, EMBASE) was conducted to identify articles published in the English language.
Study selection
Studies were included for review if: (1) more than 50% of the sample size had suffered a traumatic or non-traumatic SCI; (2) there were more than three subjects; (3) subjects received continuous intrathecal baclofen via an implantable pump aimed at improving spasticity; and (4) all subjects were ≥6 months post-SCI, at the time of the intervention.
Data extraction
Data extracted from the studies included patient and treatment characteristics, study design, method of assessment, and outcomes of the intervention.
Data synthesis
Methodological quality was assessed using the PEDro for randomized-controlled trials (RCTs) and the Downs and Black (D&B) tool for non-RCTs. A level of evidence was assigned to each intervention using a modified Sackett scale.
Conclusion
The literature search resulted in 677 articles. No RCTs and eight non-RCTs (D&B scores 13–24) met criteria for inclusion, providing a pooled sample size of 162 individuals. There was substantial level 4 evidence that intrathecal baclofen is effective in reducing spasticity. Mean Ashworth scores reduced from 3.1–4.5 at baseline to 1.0–2.0 (P < 0.005) at follow-up (range 2–41 months). Average dosing increased from 57–187 µg/day at baseline to 218.7–535.9 µg/day at follow-up. Several complications from the use of intrathecal baclofen or pump and catheter malfunction were reported.
Keywords: Baclofen, Muscle spasticity, Spinal cord injuries, Activities of daily living, Ashworth scale
Introduction
Spasticity and muscle spasms are some of the most common secondary complications following a complete or incomplete spinal cord injury (SCI). Spasticity is characterized by an increase in velocity-dependent muscle tone. Muscle spasms are spontaneous muscle contractions that are often painful and can be elicited by stretch or cutaneous stimulation. Depending on the severity, muscle spasms can interrupt an individual's ability to perform activities of daily living such as feeding, dressing, transferring, and toileting. They can also pose additional safety concerns to the individual, such as increasing the risk of falls from a wheelchair.1
Varying forms of pharmacological therapies have been trialed with the SCI population in an attempt to control spasticity and muscle spasms. Drug agents including phenol and botulinum toxin nerve blocks, clonidine, 4-aminopyridine, cyproheptadine, gabapentin, orphenadrine citrate, and cannabinoids have been found to be widely variable in their effectiveness.2 To date, the most commonly chosen drug is baclofen, a derivative of gamma-aminobutyric acid (GABA).1 Baclofen functions as an inhibitory neurotransmitter, binding to GABA-B receptors, causing membrane hyperpolarization and restriction of calcium influx to presynaptic nerve terminals. This inhibits the release of excitatory neurotransmitters and monosynaptic and polysynaptic reflexes resulting in reduced muscle contractions, thereby reducing muscle spasms.3
Baclofen can be administered either orally or intrathecally. Oral baclofen has a half-life of ∼1–5 hours and requires high daily dosages. The alternative to oral intake, intrathecal baclofen, makes use of an implanted programmable pump. Since intrathecal baclofen delivers the drug directly to the cerebral spinal fluid, lower dosages are required; this helps to minimize the often negative side effects of high oral doses.4 The half-life of continuous, steady-state infusion of intrathecal baclofen has not been determined.5 Coffey et al.6 reported that abrupt discontinuation of intrathecal baclofen may elicit adverse effects such as fatigue, drowsiness, confusion, and “intrathecal baclofen withdrawal syndrome” (e.g. seizures, death).
Intrathecal baclofen has been shown to be effective at varying time points post-injury;7 however, effectiveness specifically during the chronic stage (≥6 months) has been largely understudied. Therefore, it is important to review all of the data on intrathecal baclofen pumps for the treatment of spasticity in patients with chronic SCI. Long-term use of baclofen is an important area of concern as individuals with SCIs are living longer and there is considerable risk for side effects. Additionally, given the constraints on most health care systems, the effectiveness of such procedures should be evaluated from both a long-term effectiveness and a cost–benefit perspective. Although spasticity may sometimes be helpful for some persons with SCI in performing activities of daily living, such as those involving transfers, spasticity is mainly associated with potentially harmful consequences. Spasticity has been identified as a primary risk factor for skin breakdown, incontinence, and contractures. If impairments can continue to be minimized well after the original onset, individuals will be better able to deal with their impairments and resume personal activities as best as possible. Therefore, the objective of the current study was to review the available evidence on the effectiveness of continuous intrathecal baclofen in the treatment of spasticity during the chronic SCI stage.
Methods
Literature search strategy
A literature search of multiple databases (i.e. Pub Med, CINAHL, EMBASE) was conducted to identify articles published in the English language up to and including February 2012. Keywords included SCI, intrathecal baclofen, and spasticity. Retrieved articles were then scanned to identify relevant citations that may not have been uncovered in the original search of the databases.
Study selection
Studies were included for review if: (1) 50% or more of the sample size sustained a traumatic or non-traumatic SCI; (2) there were more than three subjects; (3) subjects received continuous intrathecal baclofen via an implantable pump aimed at improving spasticity; and (4) all subjects had an injury duration of ≥6 months, when the study intervention was initiated.
Study appraisal
Two independent reviewers assessed the selected articles for methodological quality. Randomized-controlled trials (RCTs) were assessed using the Physiotherapy Evidence Database (PEDro) scoring system; the tool consists of 11 questions with a maximum score of 10.8 Non-randomized studies were assessed using the Downs and Black (D&B) scoring system; the tool consists of 27 questions with a maximum score of 28.9 A higher score on both the PEDro and the D&B is indicative of better methodological quality.
Data synthesis
Data extracted from the studies included subject and treatment characteristics, study design, method of assessment, intervention outcomes, and adverse events. A level of evidence was assigned to each study using a modified Sackett scale. The original Sackett scale10 was composed of 10 levels of evidence; this tool was simplified to yield just five levels. Level 1 studies included RCTs with a PEDro score ≥6, whereas RCTs with a score of <6, prospective-controlled trials, and cohort studies were classified as level 2 evidence. Level 3 evidence included case control studies and level 4 evidence included pre–post studies, post-test, and case series. Finally, level 5 evidence included observational studies, clinical consensus, and case reports.
Results
Study size and quality
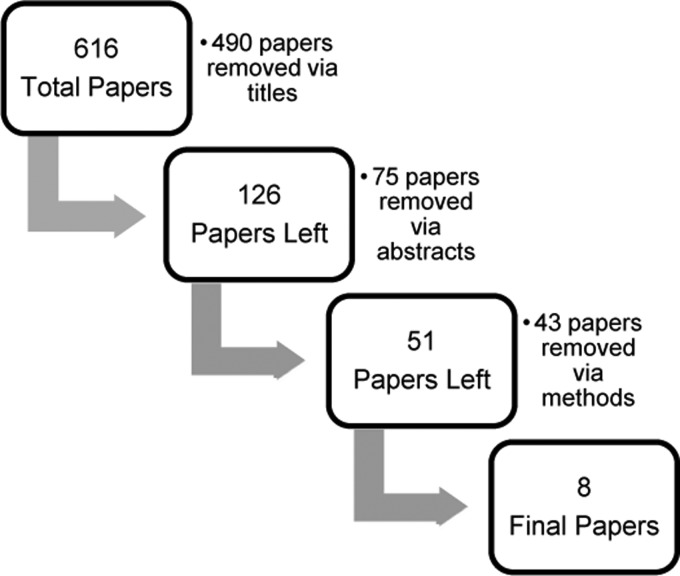
For this review on the effectiveness of intrathecal baclofen on spasticity more than 6 months post-SCI, 8 of 616 articles met inclusion criteria (Fig. 1).4,11–17 There were no RCTs that met inclusion criteria. The total pooled sample size of all included studies was 162 subjects. Sample sizes of each of the eight studies ranged from 7 to 75 with a mean of 20.3 subjects. All eight studies were rated as level 4 evidence and included pre–post and case series designs; D&B scores ranged from 13 to 24. The levels of SCI ranged from cervical to lumbar. Three older studies4,11,15 examined severity of injury using the Frankel scale, which ranged from A to D, and two studies12,16 used the ASIA impairment scale, which ranged from A to D. Two studies14,17 did not disclose the severity of their sample's injuries, whereas one study13 simply stated severity as “complete” or “incomplete”. The duration of injury ranged from 6 months to 35 years; one study13 only stipulated that they included all individuals ≥12 months post-SCI. Each study indicated that all of the subjects receiving an intrathecal baclofen pump and included for analysis were refractory to oral baclofen and/or experienced intolerable side effects from previous use of oral antispasmodics. In each of the studies, a catheter was placed into the lumbar subarachnoid space and tunneled subcutaneously into a pocket of the abdominal wall where the baclofen pump sat on either the left or right side, in the upper or lower quadrant. Other than treatment with intrathecal baclofen, no study administered concurrent anti-spasticity treatments to subjects. Treatment duration ranged from 2 months to 6 years (mean 18.8 months) with most subjects followed up monthly, or every 3 months.
Figure 1 .
Study selection.
Three primary outcome measures were used to evaluate spasticity: Modified Ashworth scale (MAS)/Ashworth scale (AS), spasm frequency score (SFS), and reflex score (RS). MAS/AS was the most common outcome measure and was used to assess spasticity in all eight studies. Secondary outcome measures included the functional independence measure (FIM) and subjective improvements in functionality, daily dosing, and adverse events. (Table 1)
Table 1 .
Studies reporting spastic and functional outcomes after treatment with intrathecal baclofen after 6 months post-SCI
| Study score research design total sample size | Subject characteristics | Treatment and follow-up | Outcome measures | Results |
|---|---|---|---|---|
| Azouvi et al.11 D&B score = 24 Pre–post n = 18 |
SCI (n = 14); mean age: 38.5 years (range 21–59); mean time post-injury: 5.7 years; level of injury: C5–T11; severity of injury: Frankel A–D | Intrathecal baclofen pump implantation followed for 9–72 months | Ashworth scale | ++ |
| spasm frequency score | ++ | |||
| Functional independence measure | ++ | |||
| Burns and Meythaler12 D&B score = 20 Case Series n = 14 |
SCI (n = 11); mean age: 41.4 years (range 25–64); time post-injury: 6 months −24 years; level of injury: C4–7; severity of injury: ASIA: A–D | Intrathecal baclofen pump implantation followed up at 1, 3, 6, 9, and 12 months post-implantation | Ashworth scale UE and LE | ++ |
| Spasm frequency score UE | + | |||
| Spasm frequency score LE | ++ | |||
| Reflex score UE and LE | ++ | |||
| Nance et al.4 D&B = 20 Pre–post n = 7 |
SCI (n = 5); mean age: 38.7 years (range 34–46); time post-injury: 4–23 years; level of injury: C5–T8; severity of injury: Frankel grade: A–B | Intrathecal baclofen pump implantation followed for 24–41 months | Modified Ashworth scale | ++ |
| Spasm frequency score | ++ | |||
| Meythaler et al.13 D&B score = 19 Pre–post n = 10 |
SCI (n = 5); mean age: 40.7 years (range 24–62); time post-injury: ≥12 months; level of injury: C/T; severity of injury: four complete, one incomplete | Intrathecal baclofen pump implantation followed monthly for 12 months | Ashworth scale | ++ |
| Spasm frequency score | ++ | |||
| Reflex score | ++ | |||
| Coffey et al.14 D&B = 18 Pre–post n = 75 |
SCI (n = 47); mean age: 42.1 years (range 25–69); time post-injury: ≥12 months; level of injury: C–L; severity of injury: unknown | Intrathecal baclofen pump implantation followed monthly for 5–41 months | Ashworth scale | + |
| Spasm frequency score | + | |||
| Loubser et al.15 D&B score = 17 Pre–post n = 7 |
SCI (n = 7); mean age: 42.4 years (range 22–61); time post-injury: 6–69 months; level of injury: C2–T12; severity of injury: Frankel A–D | Intrathecal baclofen pump implantation followed monthly for 5–24 months | Ashworth scale | ++ |
| Reflex score | ++ | |||
| Abel & Smith16 D&B score = 13 Pre–post n = 19 |
SCI (n = 15); mean age: 32.1 years (range 21–55); time post-injury: 1–35 years; level of injury: C4–T12; severity of injury: AISA: A–D | Intrathecal baclofen pump implantation followed for 2–34 months | Ashworth score | + |
| Spasm frequency score | + | |||
| Parke et al.17 D&B score = 13 Pre–post n = 8 |
SCI (n = 4); mean age: 37.8 years (range 22–61); time post-injury: 3–17 years; level of injury: C7–T12; severity of injury: unknown | Intrathecal baclofen pump implantation followed up at 3 and 6 months post-implantation | Ashworth scale | + |
| Patient evaluation conference system | + |
+, trend of improvement but not significant or no statistical analysis conducted; ++, statistically significant improvement; LE, lower extremity; UE, upper extremity.
Modified Ashworth scale/Ashworth scale
The MAS and the AS are used to assess spasticity and are widely used as clinical assessment tools. The total Ashworth score is found by summing grades for hip flexion, hip abduction, knee flexion, and ankle dorsiflexion on each side, then dividing by eight. The two scales measure resistance to passive stretch with a rating of 0–4, with 0 indicating no increase in tone and 4 indicating that the affected region is rigid in flexion or extension.18 The AS is a five-point scale (0–4) and the MAS is a six-point scale (addition of 1+ category).19 The MAS is, as its name suggests, a modified version of the AS and only one study4 used this specific method of measure. Overall, MAS scores decreased from 3.84 ± 0.23 before pump implantation to 1.80 ± 0.32 after implantation (P < 0.005). Coffey et al.14 demonstrated a decrease in Ashworth scores from 3.9 before to 1.7 after implantation; however, no statistical analysis was performed. Azouvi et al.11 reported a statistically significant decrease in Ashworth scores at 6 months post-treatment (Z = −3.79; P < 0.001). During an initial intrathecal baclofen bolus treatment for subjects, Abel and Smith16 reported that AS decreased from 3.8 before the bolus to 1.5 after. Although no further statistical analysis was conducted, the authors reported that AS remained reduced to ≤2 points even after pump implantation in all but three subjects. Loubster et al.15 reported that AS scores decreased from 3.79 ± 0.69 before to 2.00 ± 0.96 after implantation (P < 0.001). Similarly, Meythaler et al.13 reported a decrease in scores from an average of 4.36 ± 0.73 before to 2.04 ± 0.66 after implantation (P < 0.0001). Burns and Meythaler12 reported scores separately for upper and lower extremities. A significant decrease from 3.1 ± 1.3 pre-treatment to 1.7 ± 0.9 post-treatment was observed for lower extremities (P < 0.0001). Although a decrease from 2.3 ± 1.6 to 0.5 ± 0.9 was observed for upper extremities, this trend was not significant (P = 0.25). Parke et al.17 provided no statistical results, but did report that all subjects showed an improvement in Ashworth scores.
Spasm frequency score
The SFS measures the number of sustained flexor and extensor muscle spasms in 1 hour on a 0–4 rating scale with 0 indicating no spasms and 4 indicating >10 spontaneous spasms per hour.16 Nance et al.4 reported a SFS decrease from 3.50 ± 0.19 before implantation to 0.86 ± 0.14 after implantation (P < 0.005). Meythaler et al.13 also found that SFS scores decreased pre-treatment (3.30 ± 0.73) to post-treatment (1.65 ± 0.81; P < 0.0001). Azouvi et al.11 reported a statistically significant decrease in SFS at 6 months (Z = −3.79; P < 0.001). Burns and Meythaler12 determined SFS for upper and lower extremities. An insignificant decrease was observed for upper extremities with scores decreasing from 2.3 ± 1.6 before to 0.5 ± 0.9 after implantation (P = 0.2503). However, there was a significant decreased in lower extremity SFS scores from 3.3 ± 0.9 to 1.8 ± 1.5 (P = 0.0011). Coffey et al.14 had similar results with the SFS decreasing from 3.1 before implantation to 1.0 after implantation. Lastly, Abel and Smith16 reported SFS scores that decreased from 3.5 pre-bolus to 1.2 post-bolus. For the latter two studies, a statistical analysis was not performed.
Reflex score
RSs are calculated by summing the scores from knee and ankle reflexes on each side of the body and then dividing by four; this gives a number relating to the description of the reflex response.15 Three studies used this assessment tool to examine changes in spasticity. Loubster et al.15 determined that, overall, subjects’ RS decreased from 3.85 ± 0.62 before to 2.18 ± 0.43 (P < 0.001) after implantation. Burns and Meythaler12 also found that RS decreased for both upper extremities (2.3 ± 0.2 to 0.9 ± 0.2; P < 0.0001) and lower extremities (2.8 ± 1.3 to 0.4 ± 0.9; P < 0.0001). Finally, Meythaler et al.13 reported that RS decreased from 4.40 ± 0.67 before implantation to 2.18 ± 0.75 after (P < 0.0001).
Functional improvement
The FIM is an assessment of physical and cognitive disability. The assessment tool consists of 23 items that examine seven areas of function; a final score determines an individual's degree of independence, sometimes characterized as caregiver burden.11 Azouvi et al.11 reported that the average FIM scores for their sample of persons with long-standing SCI (i.e. mean time post-injury = 5.5 years) was 39.9 ± 18.1 before treatment and 58.5 ± 28.7 at 6 months post-treatment (Z = −3.62; P < 0.001). The improvement in FIM scores was statistically significant for all individual items, excluding eating and stair climbing since most people could eat independently and only two individuals could stair climb before treatment. No differences from baseline to 6 months were observed for the cognitive sub-score. The most drastic improvement was demonstrated by the items bathing, dressing the lower body, and transfers. Azouvi et al.11 also reported that for individuals who were severely disabled, nursing care was easier for attendants post-implantation, as it reduced the time and effort required to complete tasks.
Although the other studies included for this review did not assess function with the FIM, many subjective functional improvements were noted. Loubster et al.15 reported that for three subjects who were previously unable to travel, after treatment with intrathecal baclofen they could participate in extended vacations. One subject reported increased sexual functioning while another became independent with driving and was thus able to function better in the community by seeking vocational opportunities. Nance et al.4 also reported subjective improvements of all subjects in their comfort and activities of daily living. One subject who had not walked in 5 years was able to do so with the assistance of a functional electrical stimulation system. Additionally, two subjects healed their chronic ankle ulcers. Similarly, Parke et al.17 found that among three subjects, all experienced improvements in skin integrity. One subject was able to have her indwelling catheter permanently removed as she was no longer incontinent. Finally, one subject was relieved of severe pain secondary to spasticity.
Daily dosing
With the exception of Parke et al.17 the daily dose of intrathecal baclofen required to maintain the therapeutic effect increased in all of the studies, from the start of the study to its completion. The average range of increase in dosage was 127.2–343.6 µg/day. Azouvi et al.11 reported the lowest change in dosage (169.3 µg/day), whereas Loubster et al.15 reported the highest change in dosage (372.0 µg/day) from pre- to post-treatment.
Regarding intrathecal baclofen tolerance, Nance et al.4 did not indicate if any subject developed tolerance to the drug. Parke et al.17 found that only one subject developed tolerance; spastic relief could not be achieved despite the fact that the subject was on a very high dose (1600 µg/day). Meythaler et al.13 Loubster et al.15 and Burns and Meythaler12 reported that among their samples, a consistent increase in tolerance developed up to 12 months post-implantation. However, the authors of these studies reported that no subject developed complete tolerance or required drug holidays. Azouvi et al.11 noted that 14 subjects (77%) developed some level of tolerance, with a statistically significant difference between their baseline and 6-month post-implantation dosage (P < 0.001). However, these subjects did not require drug holidays. Although dosage increased between 6 and 12 months post-implantation, this trend was not significant; no dosage increase was necessary 12 months post-implantation. Similarly, Abel and Smith16 reported that among their sample, tolerance to the drug increased slowly up to 6 months post-implantation; however, this tolerance reached a plateau 6–12 months post-implantation. Only 15.8% (n = 3) of individuals developed complete tolerance which required drug holidays. During these abstinence periods, two subjects were given morphine and then returned to their initial post-implantation baclofen dose with good effect. One subject refused a drug holiday and remained on a high level of baclofen throughout the study period. Coffey et al.14 found that only 8% of their sample (n = 6) developed an episode of complete tolerance 3–31 months post-implantation; one subject had two episodes of tolerance. When treated with drug holidays of morphine, saline, or hydromorphone, baclofen was resumed at lower doses in five cases and higher doses in two cases. Thus in total, of 162 individuals included in this review, 10 developed complete tolerance and 7 of these experienced positive results after taking a drug holiday. Upon further analysis, Azouvi et al.11 reported that tolerance was not significantly associated with level of injury, severity of injury, or injury etiology.
Adverse events
With the exception of Burns and Meythaler12 and Parke et al.17 complications were largely reported and ranged from mild to severe. There were no deaths related to intrathecal baclofen treatment reported in any of the studies. Several technical issues related to catheter equipment were reported (e.g. dislodgement, kinking, breaking, etc.).11,13–16 A total of three subjects experienced seizures, one due to rapid withdrawal of baclofen from a catheter malfunction, whereas the other two were reported as unrelated to baclofen treatment. Infections and seromas at the pump incision site were also reported.4,11,14,16 Finally, three subjects experienced baclofen overdose, which was reported to be related to a mistake in pump management, and pump failure. These overdoses were characterized by somnolence, muscle flaccidity, drowsiness, and dizziness.16
Discussion
There is substantial, but only level 4, evidence based on eight studies that intrathecal baclofen is effective in the treatment of spasticity among persons with SCIs beginning 6 months post-injury or diagnosis. It should be noted that a higher level of evidence may be difficult to achieve due to the ethical and logistical constraints associated with conducting controlled studies for long-term treatment that involves invasive procedures and also for which the study population has been refractory to other forms of treatment. Several RCTs have been conducted to test single-bolus administrations of intrathecal baclofen, the same procedure for testing the viability of this treatment prior to pump implantation; these investigations have provided level 1 evidence for the short-term benefits of intrathecally delivered baclofen.2 In the studies selected for review which included individuals with chronic SCI, it was reported that the mean Ashworth scores were significantly reduced from baseline to follow-up (range 2–41 months; P < 0.005). The reduction in Ashworth scores suggest that per day, individuals experienced less muscle spasticity as a result of receiving intrathecal baclofen. The average SFS was also reduced from baseline to follow-up, signifying that the average number of spasms endured per hour declined; a reduction in total RSs substantiated this improvement in spasticity. Despite including some patients with etiologies other than SCI, three of seven studies reported that there was no significant difference in spasticity outcomes among individual with SCI and other etiologies (e.g. multiple sclerosis).13,14,16 While the remaining studies4,11,12,17 did not report on this relationship, more than 50% of the individuals in each sample had sustained a traumatic or non-traumatic SCI.
Reducing spasticity for persons with SCIs can improve their quality of life and overall function in daily activities. Azouvi et al.11 demonstrated an improvement in FIM scores from baseline to 6 months post-treatment (Z = −3.62; P < 0.001); further, a significant improvement in activities of daily living was noted. Many other improvements in physical functioning were reported by the other studies related to bladder/bowel functioning, skin integrity, transfers, and the ability to participate in the community. Other studies, not included in this review, examining the effect of spasticity on function have demonstrated improvements over the short term. Plassat et al.20 found that intrathecal baclofen distinctly improved the ease of nursing care for severely disabled individuals, along with ease of sitting and transfer capacities for wheelchair users. Furthermore, Plassat et al.20 reported a dramatic improvement in the quality of life of their patients. The medication provided patients with a sense of optimism and hope during their long-term recovery process. These results suggest that intrathecal baclofen had a positive impact for patients and may provide the basis for functional improvement.
A noteworthy concern of this study is the gradual increase in intrathecal baclofen dosage over time from 57–187 µg/day at baseline to 218.7–535.9 µg/day at follow-up. Daily doses of 1500 µg are considered extreme and typically representative of tolerance.21 Studies suggest that tolerance may be the result of a down-regulation of GABA-B receptors.22 Despite the fact that this treatment has been shown to be effective, dosage often needs to be increased over time so that individuals experience continued consistent results. Creedon et al.7 report that dosages typically accelerate immediately following the post-implantation period and for individuals whose initial dose is less than 100 µg/day. This review has found that dosage increases typically occurred up to 6 months post-pump implantation, then rose more slowly over time, and finally reached a plateau at ∼12 months. Although Akman et al.22 also found that baclofen dosing leveled-off at 12 months post-implantation, further studies are needed to better elucidate this trend. To combat baclofen resistance, Abel and Smith16 discuss “drug holidays”, a method used to interrupt the trend of accelerated dosage by weaning an individual off of the baclofen, providing an alternate drug (e.g. morphine) for a period of 4–6 weeks and then restarting treatment with intrathecal baclofen.7 Abel and Smith,16 and several other authors, have found this method to be successful, with some patients returning to a dose similar to their initial post-implantation dose.
While pump implantation was tolerated well and there were no deaths reported, there were still a high number of baclofen- or equipment-related complications and adverse events. However, few patients had to discontinue treatment. Many adverse events such as infection, catheter malfunctioning, and seromas were easily resolved. These complications were subjectively described as mild and were reported to be tolerable in comparison with the relief in spasticity that the treatment brought. Future studies should aim to report side effects in combination with individuals’ medical history (e.g. chronic health conditions) so that selection criteria for pump implantation can be improved. This would allow health care providers to better select candidates for the treatment.
Administration of intrathecal baclofen may be beneficial and the preferred option for patients who do not wish to undergo irreversible surgery (e.g. longitudinal myelotomy, Creedon et al.7), which is considered a rare, last resort for relief. In general, patients who are considered as candidates for pump implantation are those with decreased spasticity, as measured by a two-point or more decrease in AS score, for a period of 4–8 hours following an initial bolus injection of up to 100 µg baclofen.7,14 Based on Ontario Medical Advisory Secretariat23 data, the pump required for intrathecal baclofen application costs, on average, US$11,502.30 and lasts for 5–7 years before requiring replacement. The total cost per treated patient for the initial, professional costs of setup of the pump is ∼US$1300.26. The total first year cost including pump set-up and hospitalization fee is US$24,004.80. Despite the large upfront costs for the procedure, the long-term effects can be potentially money-saving. Nance et al.4 demonstrated in a cost analysis that, as a result of intrathecal baclofen, spasticity relief lead to a reduction in the number of hospitalization stays when comparing 2 years of spasticity-related hospitalization post-implantation to the 2 years prior to receiving the baclofen pump. The authors reported a net savings of ∼US$25,520.00 per person in just 2 years. While this study did not assess other health indicators, such as pharmaceuticals and personal care service costs, nor did it assess overall economic impact, it should be realized that there are potentially substantial cost savings over one's lifetime as a result of pump implantation.
Limitations
As with many older studies, some data were not provided for all outcome measures included in this review. Additionally, some articles did not report, or did not report fully, on adverse events associated with intrathecal baclofen or the medication equipment. It is important to be aware of all potential medical issues that may arise as a result of using intrathecal baclofen. The time of follow-up is another area of concern. Some authors followed their patients for as little as 2 months (Abel and Smith), even though it is recommended that patients are followed for at least 12 months to determine the effect of intrathecal baclofen on spasticity. Most importantly, there were no level 1, 2, or 3 studies included in this review. Thus, there have not been significant long-term studies conducted on this medication that included a control group, independent observers, and blind assessment. Despite lower methodological quality, the information gleaned from the level 4 studies is important in showing the beneficial use of intrathecal baclofen among individuals with chronic SCI experiencing disabling spasticity.
Conclusion
This study has demonstrated that there is a substantial quantity of research; however, there is only level 4 evidence that intrathecal baclofen is effective in reducing spasticity among individuals who were at least 6 months post-SCI. Future criteria for pump implantation should take into account the patients who require a high initial bolus dosage necessary for a therapeutic response, as these individuals will require the greatest increase in dosage over time. Since there is considerable risk for adverse events should an individual accept a pump implantation, consideration of all available treatment options is warranted. While intrathecal baclofen has improved function and quality of life among those with chronic SCI, the risk for adverse events is worth considering for those interested in this treatment. Intrathecal baclofen delivery is safe and effective for most patients; however, it should be reserved for those who are refractory to conservative treatments of medication (e.g. oral baclofen) and physical therapy. Since there was considerable difficulty attaining higher level evidence for support in using intrathecal baclofen, future research could make use of existing large-scale registries that follow cohorts of patients over long periods of time. The production of RCTs with high methodological quality (level 1 evidence) is more likely to promote the use of intrathecal baclofen in areas where this medication is not typically available. Additionally, studies that assess the cost–benefit of intrathecal baclofen using various health utility measures (e.g. Health Utilities Index, Disability Adjusted Life Years, etc.) and patient-reported outcomes (e.g. quality of life) might be useful.
References
- 1.Rabchevsky AG, Kitzman PH. Latest approaches for the treatment of spasticity and autonomic dysreflexia in chronic spinal cord injury. Neurotherapeutics 2011;8(2):274–82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hsieh JTC, Wolfe DL, McIntyre A, Janzen S, Townson AF, Short C, et al. Spasticity following spinal cord injury. In: Eng JJ, Teasell RW, Miller WC, Wolfe DL, Townson AF, Hsieh JTC, et al. (eds.) Spinal cord injury rehabilitation evidence (SCIRE; version 4). London, Ontario: SCIRE; 2012. p. 299–306 [Google Scholar]
- 3.Young RR, Delwaide PJ. Drug therapy: spasticity (second of two parts). N Engl J Med 1981;304(2):96–9 [DOI] [PubMed] [Google Scholar]
- 4.Nance P, Schryvers O, Schmidt B, Dubo H, Loveridge B, Fewer D. Intrathecal baclofen therapy for adults with spinal spasticity: therapeutic efficacy and effect on hospital admissions. Can J Neurol Sci 1995;22(1):22–9 [DOI] [PubMed] [Google Scholar]
- 5.Novartis Australia. Lioresal® Intrathecal (baclofen). North Ryde, NSW: NOVARTIS Pharmaceuticals Australia Pty Limited; 2012 [Google Scholar]
- 6.Coffey RJ, Edgar TS, Francisco GE, Graziani V, Meythaler JM, Ridgely PM, et al. Abrupt withdrawal from intrathecal baclofen: recognition and management of a potentially life-threatening syndrome. Arch Phys Med Rehabil 2002;83(6):735–41 [DOI] [PubMed] [Google Scholar]
- 7.Creedon SD, Dijkers MP, Hinderer SR. Intrathecal baclofen for severe spasticity: a meta-analysis. Int J Rehabil Health 1997;3(3):171–85 [Google Scholar]
- 8.Moseley AM, Herbert RD, Sherrington C, Maher CG. Evidence for physiotherapy practice: a survey of the Physiotherapy Evidence Database (PEDro). Aust J Physiother 2002;48(1):43–9 [DOI] [PubMed] [Google Scholar]
- 9.Downs SH, Black N. The feasibility of creating a checklist for the assessment of the methodological quality both of randomised and non-randomised studies of health care interventions. J Epidemiol Community Health 1998;52(6):377–84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Straus SE, Richardson WS, Glasziou P, Haynes RB. Evidence-based medicine: how to practice and teach EBM. Toronto, Ontario: Elsevier Churchill Livingstone; 2005 [Google Scholar]
- 11.Azouvi P, Mane M, Thiebaut JB, Denys P, Remy-Neris O, Bussel B. Intrathecal baclofen administration for control of severe spinal spasticity: functional improvement and long-term follow-up. Arch Phys Med Rehabil 1996;77(1):35–9 [DOI] [PubMed] [Google Scholar]
- 12.Burns AS, Meythaler JM. Intrathecal baclofen in tetraplegia of spinal origin: efficacy for upper extremity hypertonia. Spinal Cord 2001;39(8):413–9 [DOI] [PubMed] [Google Scholar]
- 13.Meythaler JM, Steers WD, Tuel SM, Cross LL, Haworth CS. Continuous intrathecal baclofen in spinal cord spasticity. Am J Phys Med Rehabil 1992;71(6):321–7 [DOI] [PubMed] [Google Scholar]
- 14.Coffey RJ, Cahill D, Steers W, Park TS, Ordia J, Meythaler J, et al. Intrathecal baclofen for intractable spasticity of spinal origin: results of a long-term multicenter study. J Neurosurg 1993;78(2):226–32 [DOI] [PubMed] [Google Scholar]
- 15.Loubster PG, Narayan RK, Sandin KJ, Donovan WH, Russell KD. Continuous infusion of intrathecal baclofen: long-term effects on spasticity and spinal cord injury. Paraplegia 1991;29(1):48–64 [DOI] [PubMed] [Google Scholar]
- 16.Abel NA, Smith RA. Intrathecal baclofen for treatment of intractable spinal spasticity. Arch Phys Med Rehabil 1994;75(1):54–8 [PubMed] [Google Scholar]
- 17.Parke B, Penn RD, Savoy SM, Corcos D. Functional outcome after delivery of intrathecal baclofen. Arch Phys Med Rehabil 1989;70(1):30–2 [PubMed] [Google Scholar]
- 18.Ashworth B Preliminary trial of carisoprodol in multiple sclerosis. Practitioner 1964;192:540–2 [PubMed] [Google Scholar]
- 19.Bohannon RW, Smith MB. Interrater reliability of a modified Ashworth scale of muscle spasticity. Phys Ther 1987;67(2);206–7 [DOI] [PubMed] [Google Scholar]
- 20.Plassat R, Perrouin Verbe B, Menei P, Menegalli D, Mathe JF, Richard I. Treatment of spasticity with intrathecal baclofen administration: long-term follow-up, review of 40 patients. Spinal Cord 2004;42(12):686–93 [DOI] [PubMed] [Google Scholar]
- 21.Ochs G Intrathecal baclofen. In: Ward CD, (ed.) Rehabilitation of motor disorders. London: Baillere Tindall: Baillere's Clinical Neurology; 1993. p. 73–86 [PubMed] [Google Scholar]
- 22.Akman MN, Loubser PG, Donovan WH, O'Neill ME, Rossi CD. Intrathecal baclofen: does tolerance occur? Paraplegia 1993;31(8):516–20 [DOI] [PubMed] [Google Scholar]
- 23.Medical Advisory Secretariat. Intrathecal baclofen pump for spasticity: an evidence-based analysis. Ontario Health Technol Assess Ser 2005;5(7):1–93 [PMC free article] [PubMed] [Google Scholar]