Figure 1.
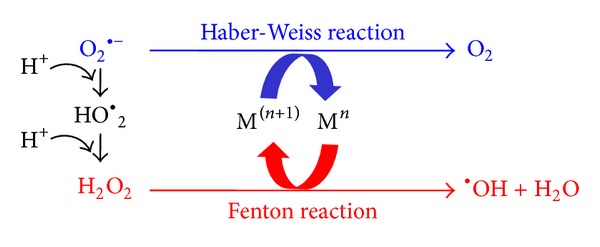
Fenton and Haber-Weiss reaction. Reduced form of transition-metals (Mn) reacts trough the Fenton reaction with hydrogen peroxide (H2O2), leading to the generation of •OH. Superoxide radical (O2 •−) can also react with oxidized form of transition metals (M(n+1)) in the Haber-Weiss reaction leading to the production of Mn, which then again affects redox cycling.
