Figure 5.
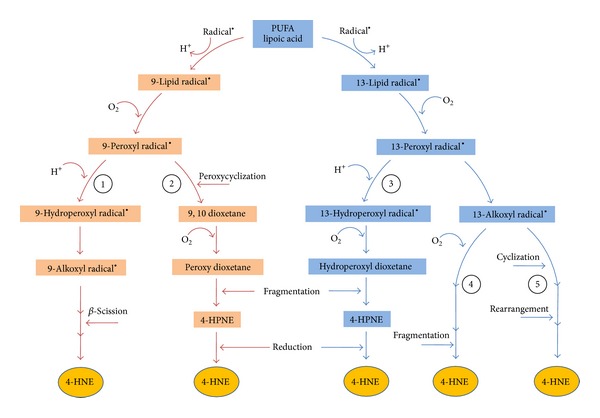
Nonenzymatic 4-HNE production. Initial abstraction of bisallylic hydrogen of lipoic acid (LA) produces fatty radicals. 4-HNE formation starting with 9- and 13-hydroperoxyoctadecadienoate (HPODE) (red and blue pathways, resp.). 4-HNE is generated by beta-scission of a hydroxyalkoxy radical that is produced after cyclization of alkoxy radical in the presence of transition metal ions and two molecules of oxygen; this reaction involves hydrogen abstraction (1). Peroxy radical cyclizes to form a dioxetane which is oxygenated to peroxy-dioxetane that is fragmented and after two hydrogen abstractions produce 4-HNE (2). Hydroperoxyl radical is oxygenated to dioxetane that is further fragmented to produce 4-hydroperoxy-2E-nonenal (4-HPNE), an immediate precursor of 4-HNE (3). Bicyclic endoperoxides react with reduced form of transition metal, such as iron (Fe2+) to produce alkoxyl radicals which after reaction with oxygen (O2), hydrogen abstraction (H+), and fragmentation produce 4-HNE (4). Alkoxyl radical after cyclization, oxygenation, hydrogen abstraction, oxidation of transition metal, hydrolysis, and rearrangement yields 4-HNE (5). With arachidonic acid, 11- and 15- hydroperoxyeicosatetraenoic acids (HPETE) are the precursors to form 4-HNE via the analogous mechanisms.
