Figure 2.
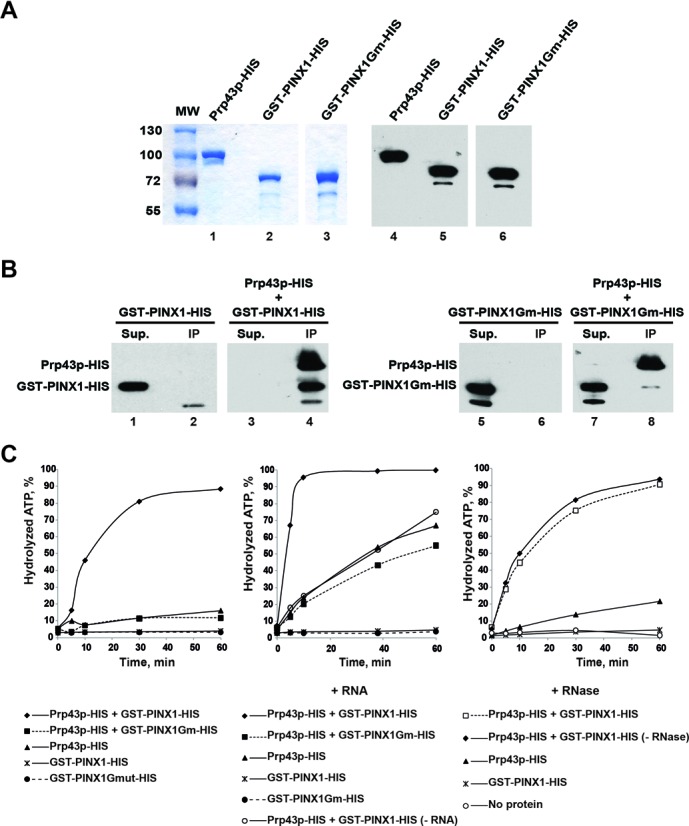
Human PINX1 binds directly to yeast Prp43p and stimulates Prp43p ATPase activity. (A) Purification and analysis of recombinant proteins. Prp43p-HIS (lanes 1 and 4), GST-PINX1-HIS (lanes 2 and 5) and GST-PINX1Gm-HIS (lanes 3 and 6) were expressed in E. coli and purified in two steps (affinity purification on a nickel column followed by size-exclusion chromatography). Purified proteins were submitted to SDS-PAGE and analyzed by Coomassie blue staining (left panel) or western using anti-histidine antibodies (right panel). (B) Pull-down assays. Purified recombinant GST-PINX1-HIS and GST-PINX1Gm-HIS were incubated with Prp43p-HIS (lanes 3–4 and 7–8, respectively). In control experiments, Prp43p-HIS was omitted (lanes 1, 2, 5 and 6). Immunoprecipitations were then carried out using anti-Prp43p antibodies. Proteins extracted from the pellets (lanes IP) or TCA precipitated from the supernatants (lanes Sup.) were subjected to SDS-PAGE, transferred to nitrocellulose membranes and detected by western using anti-histidine antibodies. (C) ATPase assays. [α-32P]-ATP was incubated with the indicated proteins and when mentioned with total yeast RNA (150 μM) or RNase (1 mg/ml). Note that assays were performed in the presence of 100-μM cold ATP, except when RNA was added, in which case the cold ATP concentration was increased to 200 μM. The percentage of hydrolyzed ATP is plotted with respect to time (in minutes).
