Figure 2.
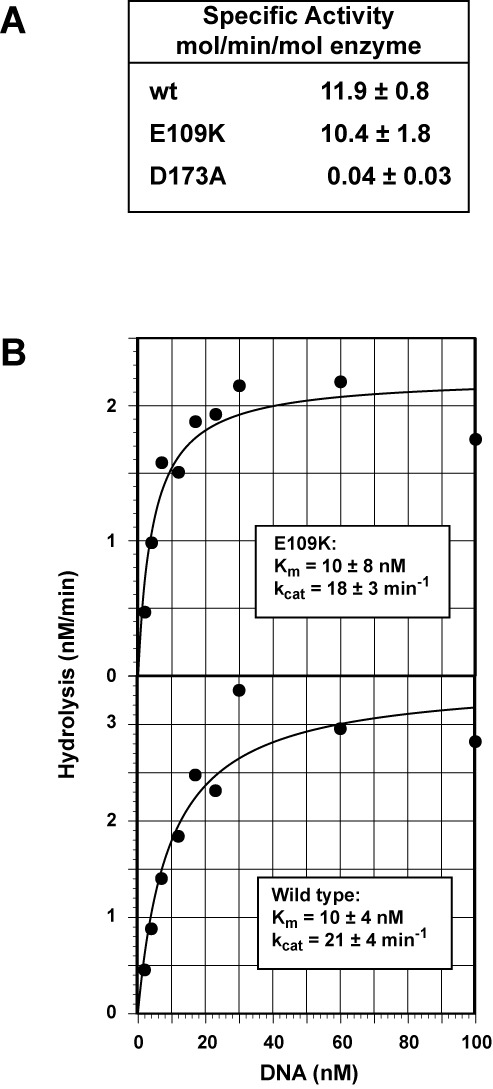
Exo1-E109K is a functional exonuclease. (A) Activities of wild type, Exo1-E109K and Exo1-D173A were determined as a function of enzyme concentration (0.01–2 nM) using a synthetic 5′-recessed 32P-labeled oligonucleotide duplex (27.5 nM, Materials and Methods). Specific activities shown were determined from progress curves where rates were linear with enzyme concentration. Results shown are the mean of 3 (wild type and Exo1-D173A) or 4 (Exo1-E109K) determinations (±one standard deviation). (B) Steady-state rates of [32P] synthetic duplex hydrolysis by 0.15 nM wild type Exo1 or Exo1-E109K were determined as a function of substrate concentration and results fit to a hyperbola using the non-linear regression function of DeltaGraph (RedRock Software). Km and kcat values shown in the insets are averages of three independent determinations (±one standard deviation).
