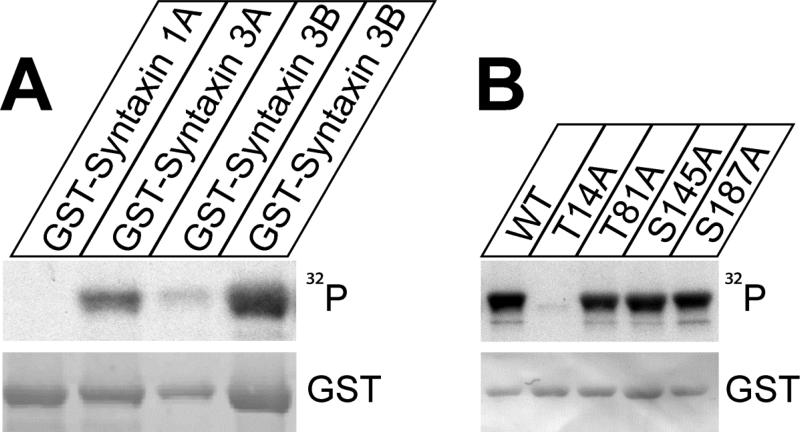
Figure 7. Syntaxin 3B is phosphorylated by CaMKII at T14.
A. GST-syntaxin fusion proteins were in vitro phosphorylated by CaMKII and Ɣ -32P ATP, separated by SDS-PAGE and analyzed by autoradiography (32P) and immunoblotting with GST antibodies. Two different amounts of syntaxin 3B were loaded to allow better comparison with the other proteins.
B: Wildtype GST-syntaxin 3B as well as mutated proteins were phosphorylated and analyzed as in A. Wild type syntaxin 3B and mutant proteins GST-syntaxin 3B T81A, S145A, and S187A were phosphorylated similarly, while the mutant protein GST-syntaxin 3B T14A was not phosphorylated, demonstrating that T14 is the phosphorylation site in syntaxin 3B.