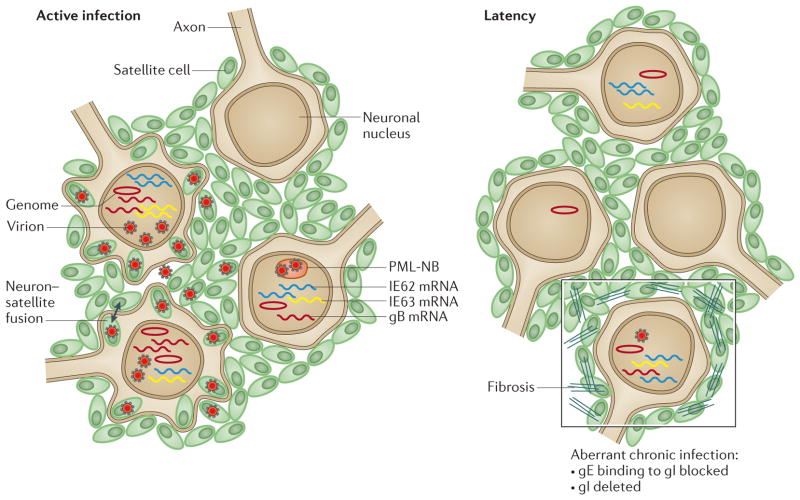
Figure 4. VZV neurotropism in DRG xenografts.
This schematic illustrates active infection of dorsal root ganglia (DRG) which is characterized by the transcription of genes (for example, genes encoding glycoprotein B (gB), immediate early protein 62 (IE62) and IE63) that produce proteins that are required for lytic infection, varicella zoster virus (VZV) genome synthesis, virus assembly in neurons and satellite cells, release of VZV into intracellular spaces and fusion of some neurons and satellite cells27 (left panel). Virions are captured in cages that are formed by promyelocytic leukaemia nuclear bodies (PML-NBs) in some neurons and satellite cells66. By contrast, latency (right panel) is associated with the persistence of VZV genomes and immediate-early (IE) transcripts, whereas late gene transcription, such as transcription of gB, ceases and virion formation ceases. When DRG are infected with VZV mutants in which binding of gE to gI is blocked or in which gI is deleted, the transition to latency is disrupted (right panel; outlined box), infectious virions continue to be produced at low levels and in the case of disrupted binding of gE to gI, tissue destruction is extensive, which is associated with disruption of the cell matrix, elimination of many neurons and the proliferation of satellite cells.