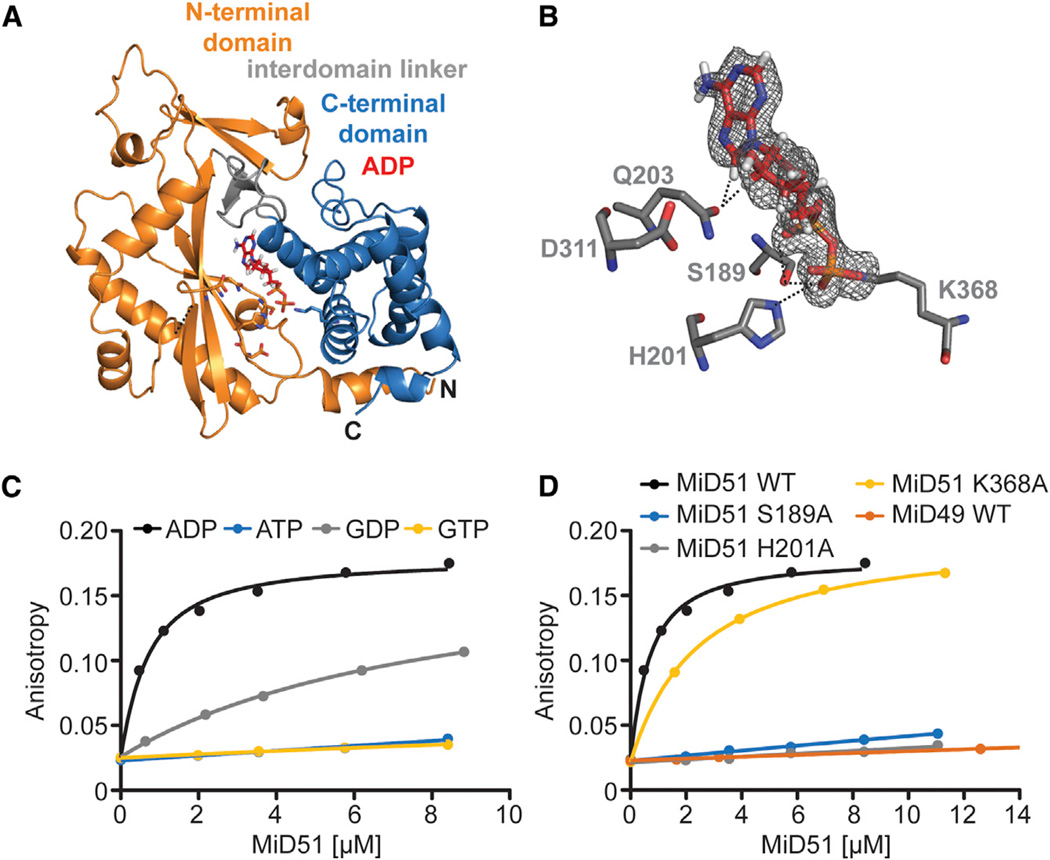
Figure 2. MiD51 Binds ADP with High Affinity.
(A) Ribbon representation of mouse MiD51Δ1–133 bound to ADP.
(B) Details of the nucleotide-binding pocket. The 2 Fo-Fc map for ADP (red) is contoured at 1.2 σ. Key residues are depicted, and interactions with the ADP are denoted with black dashed lines.
(C) Binding of MiD51 to ADP. Equilibrium titrations were performed with MANT-labeled nucleotides to determine the affinity of MiD51Δ1–133 for MANT-ATP (no binding), MANT-GTP (no binding), MANT-ADP (Kd = 0.5 µM ± 0.008 µM) and MANT-GDP (8.0 µM ± 0.9 µM).
(D) Mutational analysis of residues structurally implicated in ADP binding. Note that MiD51 S189A, H201A, and MiD49 do not bind MANT-ADP. MiD51 K368A has a Kd of 2.6 µM ± 0.4 µM.