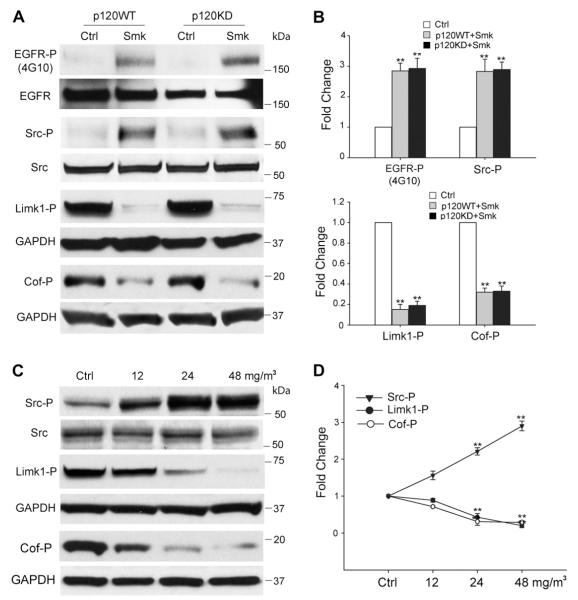
Fig. 2.
Smoke-mediated signaling events in HBE cells. (A–D) Smoke causes p120ctn-independent phosphorylation of EGFR/Src and dephosphorylation of Limk1/Cofilin (Cof) in HBE cells after 3 h of exposure. (A) Cell lysates obtained from p120WT and p120KD cells after a 3 h exposure to smoke-free (Ctrl) or smoke medium at 48 mg/m3 TSP (Smk) were analyzed by Western blot using antibodies directed against Tyr-phosphorylated EGFR (EGFR-P), Tyr416-phosphorylated Src (Src-P), Thr508-phosphorylated Limk1 (Limk1-P) and Ser3-phosphorylated Cofilin (Cof-P). Equal loading of lysates was confirmed using antibodies directed against total EGFR, Src and GAPDH. Blots are representative of three independent experiments. (B) Relative fold change in EGFR-P, Src-P, Limk1-P and Cof-P following smoke exposure in p120WT (grey bar) and 120KD cells (black bar) was normalized relative to Ctrl (white bars, designated 1-fold). Results shown are the mean ± SEM of three independent experiments. **p < 0.01, p120KD or p120WT versus Ctrl cells. (C) HBE cell lysates exposed to smoke-free (Ctrl) or smoke medium at 12, 24 and 48 mg/m3 TSP (Smk) for 3 h were analyzed by Western blot using antibodies directed against Src-P, Limk1/2-P and Cof-P. Levels of total Src and GAPDH were used as loading controls. (D) Relative fold change in Src, Limk1 and Cof phosphorylation induced by smoke was normalized to Ctrl (designated 1-fold) at 3 h and plotted as dose-response curves. Results shown are the mean ± SEM of three independent experiments. **p < 0.01, Smk-exposed versus Ctrl cells.