Abstract
Our immune system is characterized by remarkable specificity, potency and memory – the ability of a single vaccine treatment to provide life-long protection. No pharmacologic treatment for any indication can provide the same level of safety, efficacy and long-lasting effect that a vaccine can. Thus, researchers and clinicians alike have sought to apply these characteristics to the treatment of cancer. Yet, for the last 125 years, the field has failed to realize this potential. Here, we will review some of the most promising cancer immunotherapeutic approaches in development today, as recent clinical successes signal the beginning of cancer immunotherapy’s transition from experimental to established therapy.
Introduction
As the human population becomes increasingly older, the medical, economic and social burden imposed by cancer becomes increasingly greater. Indeed, cancer has surpassed heart disease as the leading cause of death in Hispanic/Latino patients in the United States (Siegel et al., 2012). While several socioeconomic characteristics of the US Hispanic population contribute to this shift, cancer is expected to become the leading cause of death in the US across populations, making the need for therapeutics greater than ever.
Mortality in cancer is typically associated with metastatic disease. For example, colorectal cancer is highly treatable with surgery to remove the primary tumor, but colorectal cancer metastases in liver, lung, and brain are almost always lethal. Therefore, conventional cancer therapy usually involves surgical removal of the primary tumor followed by adjuvant therapy to treat metastatic disease. Metastases may be detected early as micrometastases in draining lymph nodes by histopathology or they may be larger, distant growths. The objective of treating micrometastases is to prevent them from spreading to distant organs and growing into large and lethal masses. In contrast, large, distant metastases are treated more aggressively to try to eliminate the disease. Unfortunately, many cases of distant metastases involve management of the patient, rather than treatment, reflecting the terminal nature of the disease. Therefore, cancer diagnoses and prognoses typically fall into one of three types: local (highly treatable), local with micrometastases (marginally treatable), and local with distant metastases (untreatable). Discovery and design of new therapeutics should be tailored to patient diagnosis and prognosis, a concept that we will revisit.
Vaccines
The idea of employing the immune system to treat cancer was formally introduced by William Coley in 1893 (Coley, 1893), though the cellular and molecular mechanisms underlying the process were unknown. Coley leveraged the serendipitous discovery of a sarcoma patient who developed a spontaneous remission after a Streptococcus pyogenes infection (Hoption Cann et al., 2003). The patient’s tumor recurred after multiple surgeries until open surgical wounds became infected with S pyogenes. Over a period of several months the patients tumor shrank until he was deemed cured and discharged. That patient remained cancer-free several years later. Coley suspected that the infection was responsible for the patient’s remarkable recovery and he carried out a serious of experiments employing deliberate infection of cancer patients with S pyogenes, that resulted at times in failed infections, death and cancer regression. He then developed a version of his treatment containing a mixture of killed S pyogenes and Serratia marcescens. This approach became known as ‘Coley’s toxins’ and it produced several remarkable recoveries in patients with advanced disease.
Interestingly, a retrospective study was conducted to compare the 10-year survival of patients treated with Coley’s toxins to that of patients treated with modern conventional therapy (Richardson et al., 1999). Although limitations exist in this study reflecting the 100 year disparity in data collection, the results suggest that Coley’s toxins were comparable in efficacy to modern treatments. In the context of our modern understanding of immunology, tumor immunologists have developed a number of immunotherapeutic approaches for cancer that were impossible during Coley’s time. Here, we will highlight some of these, the most prominent of which has been the use of cancer vaccines.
Antigen Non-Specific Vaccines
Like conventional vaccines to infectious diseases, the objective of cancer vaccines is to produce an immune response that eliminates cancer cells and produces long-lasting immunity. Traditionally, vaccines for infectious diseases contained an inactivated (non-infectious) form of the pathogen. These would stimulate an immune response, but not risk the development of disease from the pathogen. Not surprisingly, tumor immunologists mimicked the approaches against infectious diseases and employed inactivated tumor cell-based vaccines. These could be in the form of tumor lysates, irradiated tumor cells, etc. These were advantageous because they required no knowledge of the immunogenic components of the tumors since the vaccine would theoretically contain all possible immunogens within the cancer and elicit all of the necessary responses. However, these approaches proved ineffective in clinical trials. One prominent example of this approach is an irradiated, polyvalent, whole-cell melanoma vaccine known as Canvaxin, which seemed promising in phase II studies of melanoma patients at risk for relapse (Morton et al., 1992; Morton et al., 2002). However, thorough phase III testing revealed no benefit of Canvaxin (Morton et al., 2007), ultimately leading to the discontinuation of its development (Kelland, 2006). As a result of this and other failures, tumor cell-based vaccines are not currently an approach of interest for most cancers.
Antigen-Specific Vaccines
Today, most cancer vaccine approaches utilize a specific target antigen that is expressed in the cancer. Ideally, that antigen is cancer-specific, though this ideal is rarely met. In antigen-specific vaccines, the antigen is delivered in the context of vectors (viral, DNA or cells) and adjuvants that activate the innate immune system to initiate an adaptive immune response. Here, we will focus on examples of two antigen-specific vaccine approaches, reflecting their relative efficacy compared to other approaches.
Because our immune system evolved to fight infectious diseases, it would stand to reason that camouflaging a cancer antigen vaccine as an infectious pathogen would have the greatest potential to induce immune responses. Importantly, our immune system has evolved different effector mechanisms to fight different kinds of pathogens, such that responses against bacteria, viruses, parasites and fungi are typically quite different from one another. In that context, the ideal antitumor effectors - CD8+ cytotoxic T lymphocytes (CTLs) - are most efficiently generated by viruses. Given, the relative ease to produce non-replicating, recombinant viral vectors and their propensity to produce CTLs, viral vectors are most commonly used in effective cancer vaccines. The second vaccine approach we will focus on is dendritic cell (DC) vaccines. DCs are a critical innate immune cell that acquires and “presents” antigens to T cells and are vital to the development of robust immune responses. Indeed, antigens delivered by a viral vector vaccine are acquired by DCs which present the cancer antigens and initiate responses. A DC vaccine simply avoids the vectored delivery step by delivering cancer antigens directly to DCs ex vivo, followed by re-administration of the antigen-loaded DCs back to the patient. Because both of these approaches have been employed and seen successes in prostate cancer we will focus on viral vector and DC-based vaccines for prostate cancer.
Among the antigens expressed by prostate cancer, there are two well-known proteins that initially served as prostate cancer biomarkers - prostate-specific antigen (PSA) and prostatic acid phosphatase (PAP) - and both have been utilized in prostate cancer vaccines. PROSTVAC is a pox virus-based vaccine containing PSA and a triad of costimulatory molecules [B7-1, ICAM-1, and LFA-3 (TRICOM)] within the viral vector to increase antigen-specific immune responses and clinical responses (von Mehren et al., 2001; von Mehren et al., 2000). In a recent phase II clinical trial in advanced prostate cancer patients (Kantoff et al., 2010b), overall survival after vaccination was compared between 82 patients receiving PROSTVAC and a control cohort of 40 patients receiving control vaccine. Overall-survival was significantly better in the PROSTVAC group (30%) than controls (17%), and median survival was increased 8.5 months. Moreover, an ongoing Phase 1 study of early-stage prostate cancer patients reported promising preliminary results (Nordic, 2011). In the study, 21 patients with locally recurrent prostate cancer after primary radiation therapy received PROSTVAC vaccination. Stable or improved serum PSA levels were observed in 80% of the patients, suggesting that PROSTVAC-induced immune responses prevented disease progression in these early stage patients.
Currently, the only FDA-approved cancer vaccine is sipuleucel-T (Provenge®) manufactured by Dendreon for the treatment of advanced prostate cancer (Drake, 2010). Sipuleucel-T consists of monocytes (including DCs) collected from the prostate cancer patient exposed ex vivo to a fusion protein of PAP and GM-CSF. GM-CSF is a cytokine that aids in PAP uptake by the DCs and induces DC maturation to increase T cell activation. The monocytes are then administered to the patient to activate PAP-specific CD8+ T cells. In the phase III IMPACT (IMmunotherapy Prostate AdenoCarcinoma Treatment) trial of 512 patients with metastatic castration-resistant prostate cancer, sipuleucel-T improved median overall-survival 4.1 months and reduced the risk of death by 22% compared to placebo (Kantoff et al., 2010a). While those results are modest, especially compared to PROSTVAC, sipuleucel-T has overcome the previously insurmountable barrier of safety, efficacy and FDA-approval to establish a model for successful translation of cancer vaccines to patients.
Passive Therapy
Cancer vaccines rely on the patient’s immune system to generate effective antitumor immune responses, however, this is not always ideal. Previous therapy, including chemotherapy, or ongoing diseases process can adversely affect the patient’s immune system making vaccine-induced immune responses suboptimal or impossible. Therefore, a passive immunotherapy approach can be used by administrating immunologic effectors to patients, rather than activating the patient’s immune system to produce immune effectors. These approaches include the use of antibodies or effector immune cells as passive immunotherapeutics.
Antibodies
The development of monoclonal antibody technology in the 1970s delivered a remarkable tool to researchers and clinicians by providing the ability to produce high quantities of a single antibody specific for virtually any antigen of interest (Kohler & Milstein, 1975). In cancer therapy, monoclonal antibodies are currently employed primarily as ligand-blocking molecules or as immunomodulatory agents in the treatment of cancer.
Epidermal growth factor receptor (EGFR) is frequently over-expressed in various cancers providing signals for proliferation, survival, migration, adhesion, and invasion through the MAPK/KRAS signaling pathway (Wells, 2000). The monoclonal antibodies cetuximab (Erbitux®) and panitumumab (Vecticbix™), block the interaction of EGFR with its ligand EGF, preventing EGFR activation and MAPK/KRAS signaling (Aboud-Pirak et al., 1988). Similarly, human epidermal growth factor receptor 2 (HER2) is overexpressed by ~25% of breast cancers (Her2+ tumors) and is targeted by the monoclonal antibody trastuzumab (Herceptin®) (Murphy & Morris, 2012). Both EGFR and Her2 –targeting monoclonal antibodies are FDA-approved and offer substantial patient benefit, though resistance may develop through the accumulation of mutations in downstream signaling pathways that make receptor engagement unnecessary (Beganovic, 2009; Lievre et al., 2006; Lievre et al.).
An alternative strategy to limit tumor growth has focused on blocking the inhibitory receptors on immune cells to allow the generation/expansion of endogenous antitumor responses. CD8+ T cells are major immune effectors in antitumor immunity, but they are tightly regulated by the expression of various stimulatory and inhibitory receptors. Inhibitory receptors play a vital role in preventing autoimmunity and tissue damage associated with chronic inflammatory infections (Pardoll, 2012). Monoclonal antibodies can be employed to block the engagement of these inhibitory receptors, including cytotoxic T-lymphocyte antigen 4 (CTLA-4) and programmed cell death protein-1 (PD-1) resulting in the activation of T effector cells that typically remain dormant during tumor growth. Ipilimumab (Yervoy™) alone, or in combination with a gp100 peptide vaccine, increased survival in patients with late-stage melanoma by ~3.5 months compared to patients receiving the vaccine alone (Hodi et al., 2010), leading to the recent FDA approval of ipilimumab for the treatment of late-stage melanoma (Cameron et al., 2011). Antibodies targeting the PD-1 inhibitory pathway are not yet FDA-approved but have produced objective responses in patients with non-small cell lung cancer, melanoma, and renal carcinoma (Topalian et al., 2012). Beyond these, other immunomodulatory agents targeting regulatory T cells (Tregs) (Ruter et al., 2009), myeloid-derived suppressor cells (MDSCs) (Ugel et al., 2009), TGF-β (Hanks & Morse, 2010) and others are under investigation and offer promise in combination with various immunotherapeutics in the future.
Adoptive Cell Therapy
Beyond antibodies, it is also feasible to transfer effector cells as passive immunotherapeutics, though the acquisition, manufacturing and re-administration of these therapies are significantly more difficult than that of monoclonal antibodies (Restifo et al., 2012). Initially ACT was developed to utilize tumor-infiltrating lymphocytes (TIL) from surgically resected tumors in patients with metastatic melanoma. Melanoma-specific T cells within the TIL preparation could be expanded ex vivo via co-culture with patient tumors in the presence of the cytokine IL-2 (Rosenberg et al., 2011). Re-administration of these T cell products back into the donor patients resulted in remarkable responses: 72% of patients experience objective responses and 40% of patients had complete regressions (Rosenberg, 2011). However, the process of collecting and expanding TIL is feasible only in melanoma, presumably due to lower immunogenicity of other cancers. A solution to the problem of TIL collection is collecting naïve T cells from peripheral blood and engineering them to express cancer antigen-specific receptors, an approach that would be applicable to all forms of cancer. Indeed, T cells have been engineered to express T cell receptors (TCRs) targeting the melanoma antigens MART-1 and gp100, producing objective responses in 30% and 19% of patients respectively (Johnson et al., 2009). An evolution of that approach employs chimeric antigen receptors (CARs) composed of an antigen-binding variable fragment from monoclonal antibodies fused to intracellular T cell signaling domains from CD3ζ, CD28, 4-1BB and/or other signaling molecules (Bridgeman et al., 2010). CARs are advantageous because they target native cell-surface antigens in an MHC-independent manner, allowing the generation of a universal product across patients, while TCR approaches are specific to patients or a group of patients. To date, B cell leukemias expressing the differentiation antigen CD19 have been the most widely targeted in CAR ACT. In a recent trial, six of eight patients with various forms of leukemia treated with CD19-specific CARs experienced objective responses, one of which was a complete response (Kochenderfer et al., 2011). In a separate study, all three chronic lymphocytic leukemia (CLL) patients treated with CD19-specific CARs experienced a reduction in tumor burden of at least one kilogram, and two of these patients experienced complete responses (Kalos et al., 2011). These studies demonstrate the remarkable ability of ACT to eliminate even massive tumor burdens, producing objective responses and even complete remissions.
Conclusions
The field of cancer immunotherapy has seen many highs and lows between Coley’s toxins and the myriad of approaches under development today. Indeed, it is our opinion that cancer immunotherapy is approaching a watershed moment where it transitions from the experimental to mainstream cancer treatment. However, critical to that transition is the need for understanding how to utilize all of the therapeutic tools becoming available to optimally manage patients. For example, we have seen that vaccine approaches can prolong survival in some patients with advanced disease, though mounting data suggests that vaccine approaches will be most effective in early stage patients, reflecting their lower tumor burden. In contrast, ACT has produced remarkable responses in patients with massive tumor burdens, but ACT remains costly, time consuming, and requires relatively harsh preconditioning regimens that put the patient at risk for opportunistic infections. Thus there is no universal immunotherapy that is appropriate for all patients (Figure 1). Clinicians will have to determine the right combination of vaccines, ACT, immunomodulation and conventional therapeutics for each patient. While that task is significant, we look forward to seeing the routine use of immunotherapeutics in treating cancer patients in the near future.
Figure 1. Immunotherapeutic management of patients across disease stages.
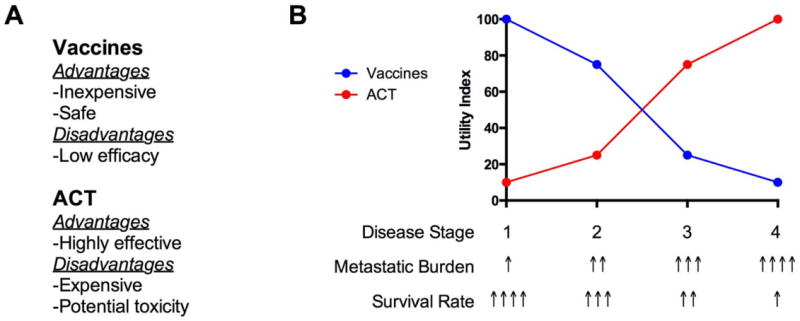
A) Vaccines and adoptive cell therapy (ACT) are associated with significant advantages and disadvantages. B) Patients are typically diagnosed with cancer in one of four stages (1-4). Metastatic burden increases with disease stage from undetectable micrometastases in early stages to large distant metastases in late stages. Reflecting the increasing metastatic burden, survival decreases sharply with increasing stage. In the context of their low cost, toxicity and decreasing efficacy with tumor burden, vaccines likely have the greatest utility in early stage patients. However, due to the high costs and treatment-associated risk to patients, ACT is inappropriate for early stage patients. Rather, ACT is ideally suited for late stage patients in which ACT has demonstrated the ability to eliminate even very large tumor burdens. Optimal patient management will come with ability to identify the appropriate combination of the available immunotherapeutics and conventional chemo/radio –therapeutics.
Acknowledgments
Support was provided by grants from the National Institutes of Health (RC1 CA146033, P30 CA56036, R01 CA170533), the Pennsylvania Department of Health (SAP #4100059197, SAP #4100051723), and Targeted Diagnostic and Therapeutics Inc. The Pennsylvania Department of Health specifically disclaims responsibility for any analyses, interpretations or conclusions. S.A.W. is the Samuel MV Hamilton Professor of Thomas Jefferson University.
Disclosure
S.A.W. is the Chair of the Data Safety Monitoring Board for the C-Cure Trial™ sponsored by Cardio3 Biosciences, and the Chair (uncompensated) of the Scientific Advisory Board of Targeted Diagnostics & Therapeutics, Inc. which provided research funding that, in part, supported this work and has a license to commercialize inventions related to this work.
References
- Aboud-Pirak E, Hurwitz E, Pirak ME, Bellot F, Schlessinger J, Sela M. Efficacy of antibodies to epidermal growth factor receptor against KB carcinoma in vitro and in nude mice. J Natl Cancer Inst. 1988;80(20):1605–1611. doi: 10.1093/jnci/80.20.1605. [DOI] [PubMed] [Google Scholar]
- Beganovic S. Clinical significance of the KRAS mutation. Bosn J Basic Med Sci. 2009;9(Suppl 1):17–20. doi: 10.17305/bjbms.2009.2749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bridgeman JS, Hawkins RE, Hombach AA, Abken H, Gilham DE. Building better chimeric antigen receptors for adoptive T cell therapy. Curr Gene Ther. 2010;10(2):77–90. doi: 10.2174/156652310791111001. [DOI] [PubMed] [Google Scholar]
- Cameron F, Whiteside G, Perry C. Ipilimumab: first global approval. Drugs. 2011;71(8):1093–1104. doi: 10.2165/11594010-000000000-00000. [DOI] [PubMed] [Google Scholar]
- Coley WB. The treatment of malignant tumors by repeated inoculations of erysipelas: with a report of ten original cases. Am J Med Sci. 1893;105:487–511. [PubMed] [Google Scholar]
- Drake CG. Prostate cancer as a model for tumour immunotherapy. Nat Rev Immunol. 2010;10(8):580–593. doi: 10.1038/nri2817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanks BA, Morse MA. Pharmacological inhibition of TGFbeta as a strategy to augment the antitumor immune response. Current opinion in investigational drugs. 2010;11(12):1342–1353. [PubMed] [Google Scholar]
- Hodi FS, O’day SJ, Mcdermott DF, Weber RW, Sosman JA, Haanen JB, Gonzalez R, Robert C, Schadendorf D, Hassel JC, Akerley W, Van Den Eertwegh AJ, Lutzky J, Lorigan P, Vaubel JM, Linette GP, Hogg D, Ottensmeier CH, Lebbe C, Peschel C, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363(8):711–723. doi: 10.1056/NEJMoa1003466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoption Cann SA, Van Netten JP, Van Netten C. Dr William Coley and tumour regression: a place in history or in the future. Postgrad Med J. 2003;79(938):672–680. [PMC free article] [PubMed] [Google Scholar]
- Johnson LA, Morgan RA, Dudley ME, Cassard L, Yang JC, Hughes MS, Kammula US, Royal RE, Sherry RM, Wunderlich JR, Lee CC, Restifo NP, Schwarz SL, Cogdill AP, Bishop RJ, Kim H, Brewer CC, Rudy SF, Vanwaes C, Davis JL, et al. Gene therapy with human and mouse T-cell receptors mediates cancer regression and targets normal tissues expressing cognate antigen. Blood. 2009;114(3):535–546. doi: 10.1182/blood-2009-03-211714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalos M, Levine BL, Porter DL, Katz S, Grupp SA, Bagg A, June CH. T cells with chimeric antigen receptors have potent antitumor effects and can establish memory in patients with advanced leukemia. Sci Transl Med. 2011;3(95):95ra73. doi: 10.1126/scitranslmed.3002842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kantoff PW, Higano CS, Shore ND, Berger ER, Small EJ, Penson DF, Redfern CH, Ferrari AC, Dreicer R, Sims RB, Xu Y, Frohlich MW, Schellhammer PF, Investigators IS. Sipuleucel-T immunotherapy for castration-resistant prostate cancer. N Engl J Med. 2010a;363(5):411–422. doi: 10.1056/NEJMoa1001294. [DOI] [PubMed] [Google Scholar]
- Kantoff PW, Schuetz TJ, Blumenstein BA, Glode LM, Bilhartz DL, Wyand M, Manson K, Panicali DL, Laus R, Schlom J, Dahut WL, Arlen PM, Gulley JL, Godfrey WR. Overall survival analysis of a phase II randomized controlled trial of a Poxviral-based PSA-targeted immunotherapy in metastatic castration-resistant prostate cancer. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2010b;28(7):1099–1105. doi: 10.1200/JCO.2009.25.0597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelland L. Discontinued drugs in 2005: oncology drugs. Expert opinion on investigational drugs. 2006;15(11):1309–1318. doi: 10.1517/13543784.15.11.1309. [DOI] [PubMed] [Google Scholar]
- Kochenderfer JN, Dudley ME, Feldman SA, Wilson WH, Spaner DE, Maric I, Stetler-Stevenson M, Phan GQ, Hughes MS, Sherry RM, Yang JC, Kammula US, Devillier L, Carpenter R, Nathan DA, Morgan RA, Laurencot C, Rosenberg SA. B-cell depletion and remissions of malignancy along with cytokine-associated toxicity in a clinical trial of anti-CD19 chimeric-antigen-receptor-transduced T cells. Blood. 2011 doi: 10.1182/blood-2011-10-384388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohler G, Milstein C. Continuous cultures of fused cells secreting antibody of predefined specificity. Nature. 1975;256(5517):495–497. doi: 10.1038/256495a0. [DOI] [PubMed] [Google Scholar]
- Lievre A, Bachet JB, Le Corre D, Boige V, Landi B, Emile JF, Cote JF, Tomasic G, Penna C, Ducreux M, Rougier P, Penault-Llorca F, Laurent-Puig P. KRAS mutation status is predictive of response to cetuximab therapy in colorectal cancer. Cancer Res. 2006;66(8):3992–3995. doi: 10.1158/0008-5472.CAN-06-0191. [DOI] [PubMed] [Google Scholar]
- Lievre A, Rouleau E, Buecher B, Mitry E. Clinical significance of BRAF mutations in colorectal cancer. Bull Cancer. 97(12):1441–1452. doi: 10.1684/bdc.2010.1225. [DOI] [PubMed] [Google Scholar]
- Morton DL, Foshag LJ, Hoon DS, Nizze JA, Famatiga E, Wanek LA, Chang C, Davtyan DG, Gupta RK, Elashoff R, et al. Prolongation of survival in metastatic melanoma after active specific immunotherapy with a new polyvalent melanoma vaccine. Ann Surg. 1992;216(4):463–482. doi: 10.1097/00000658-199210000-00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morton DL, Hsueh EC, Essner R, Foshag LJ, O’day SJ, Bilchik A, Gupta RK, Hoon DS, Ravindranath M, Nizze JA, Gammon G, Wanek LA, Wang HJ, Elashoff RM. Prolonged survival of patients receiving active immunotherapy with Canvaxin therapeutic polyvalent vaccine after complete resection of melanoma metastatic to regional lymph nodes. Ann Surg. 2002;236(4):438–448. doi: 10.1097/00000658-200210000-00006. discussion 448-439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morton DL, Mozzillo N, Thompson JF, Kelley MC, Faries M, Wagner J, Schneebaum S, Schuchter L, Gammon G, Elashoff R. An international, randomized, phase III trial of bacillus Calmette-Guerin (BCG) plus allogeneic melanoma vaccine (MCV) or placebo after complete resection of melanoma metastatic to regional or distant sites. Journal of Clinical Oncology, 2007 ASCO Annual Meeting Proceedings (Post-Meeting Edition) 2007;25(18S):8508. [Google Scholar]
- Murphy CG, Morris PG. Recent advances in novel targeted therapies for HER2-positive breast cancer. Anti-cancer drugs. 2012;23(8):765–776. doi: 10.1097/CAD.0b013e328352d292. [DOI] [PubMed] [Google Scholar]
- Nordic B. Cancer Vaccine Holds Promise In Early Stage Prostate Cancer. Medical News Today 2011 [Google Scholar]
- Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer. 2012;12(4):252–264. doi: 10.1038/nrc3239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Restifo NP, Dudley ME, Rosenberg SA. Adoptive immunotherapy for cancer: harnessing the T cell response. Nat Rev Immunol. 2012;12(4):269–281. doi: 10.1038/nri3191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson MA, Ramirez T, Russell NC, Moye LA. Coley toxins immunotherapy: a retrospective review. Alternative therapies in health and medicine. 1999;5(3):42–47. [PubMed] [Google Scholar]
- Rosenberg SA. Cell transfer immunotherapy for metastatic solid cancer-what clinicians need to know. Nat Rev Clin Oncol. 2011 doi: 10.1038/nrclinonc.2011.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg SA, Yang JC, Sherry RM, Kammula US, Hughes MS, Phan GQ, Citrin DE, Restifo NP, Robbins PF, Wunderlich JR, Morton KE, Laurencot CM, Steinberg SM, White DE, Dudley ME. Durable complete responses in heavily pretreated patients with metastatic melanoma using T-cell transfer immunotherapy. Clin Cancer Res. 2011;17(13):4550–4557. doi: 10.1158/1078-0432.CCR-11-0116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruter J, Barnett BG, Kryczek I, Brumlik MJ, Daniel BJ, Coukos G, Zou W, Curiel TJ. Altering regulatory T cell function in cancer immunotherapy: a novel means to boost the efficacy of cancer vaccines. Frontiers in bioscience: a journal and virtual library. 2009;14:1761–1770. doi: 10.2741/3338. [DOI] [PubMed] [Google Scholar]
- Siegel R, Naishadham D, Jemal A. Cancer statistics for Hispanics/Latinos, 2012. CA Cancer J Clin. 2012;62(5):283–298. doi: 10.3322/caac.21153. [DOI] [PubMed] [Google Scholar]
- Topalian SL, Hodi FS, Brahmer JR, Gettinger SN, Smith DC, Mcdermott DF, Powderly JD, Carvajal RD, Sosman JA, Atkins MB, Leming PD, Spigel DR, Antonia SJ, Horn L, Drake CG, Pardoll DM, Chen L, Sharfman WH, Anders RA, Taube JM, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med. 2012;366(26):2443–2454. doi: 10.1056/NEJMoa1200690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ugel S, Delpozzo F, Desantis G, Papalini F, Simonato F, Sonda N, Zilio S, Bronte V. Therapeutic targeting of myeloid-derived suppressor cells. Current opinion in pharmacology. 2009;9(4):470–481. doi: 10.1016/j.coph.2009.06.014. [DOI] [PubMed] [Google Scholar]
- Von Mehren M, Arlen P, Gulley J, Rogatko A, Cooper HS, Meropol NJ, Alpaugh RK, Davey M, Mclaughlin S, Beard MT, Tsang KY, Schlom J, Weiner LM. The influence of granulocyte macrophage colony-stimulating factor and prior chemotherapy on the immunological response to a vaccine (ALVAC-CEA B7.1) in patients with metastatic carcinoma. Clin Cancer Res. 2001;7(5):1181–1191. [PubMed] [Google Scholar]
- Von Mehren M, Arlen P, Tsang KY, Rogatko A, Meropol N, Cooper HS, Davey M, Mclaughlin S, Schlom J, Weiner LM. Pilot study of a dual gene recombinant avipox vaccine containing both carcinoembryonic antigen (CEA) and B7.1 transgenes in patients with recurrent CEA-expressing adenocarcinomas. Clin Cancer Res. 2000;6(6):2219–2228. [PubMed] [Google Scholar]
- Wells A. Tumor invasion: role of growth factor-induced cell motility. Adv Cancer Res. 2000;78:31–101. doi: 10.1016/s0065-230x(08)61023-4. [DOI] [PubMed] [Google Scholar]


