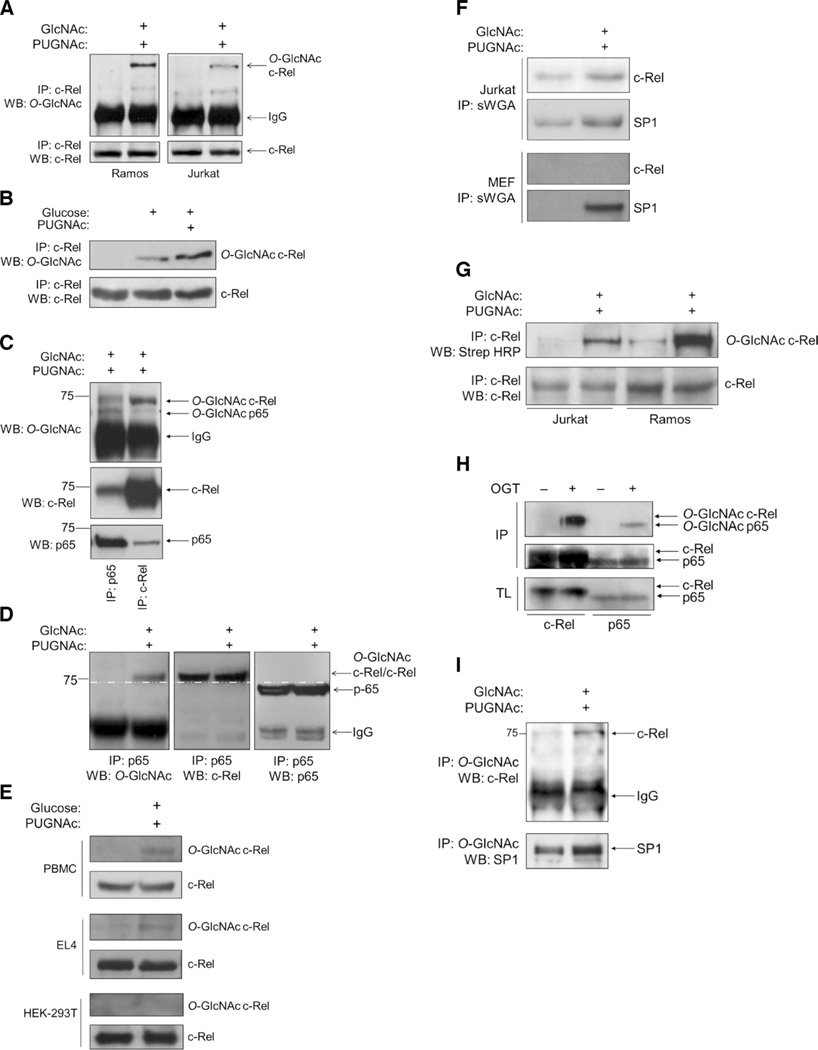
Fig. 1. The NF-κB subunit c-Rel is modified by O-GlcNAcylation.
(A) Ramos cells and Jurkat cells (50 × 106 each) were left untreated or were treated with 4 mM GlcNAc and 100 µM PUGNAc for 12 hours. Samples were subjected to immunoprecipitation (IP) with an anti–c-Rel antibody and were analyzed by Western blotting (WB) with an antibody against O-GlcNAc (RL2). (B) Jurkat cells were left untreated or were treated with 100 µM PUGNAc for 12 hours and 30 mM glucose for 3 hours or with glucose alone. Samples were analyzed as described in (A). (C) Splenocytes (75 × 106) from C57BL/6 mice were treated with 4 mM GlcNAc and 100 µM PUGNAc for 8 hours, subjected to immunoprecipitation with antibodies against p65 or c-Rel, and analyzed by Western blotting with antibodies against the indicated proteins. (D) Ramos cells were treated with 4 mM GlcNAc and 100 µM PUGNAc for 12 hours and then were subjected to immunoprecipitation with anti-p65 antibody. O-GlcNAcylation of the 75-kD protein was detected with the anti–O-GlcNAc (RL2) antibody (left). The blot was reincubated with antibodies against c-Rel and p65. c-Rel coimmunoprecipitated with p65 (middle). The p65 protein ran with a lower molecular mass than did the O-GlcNAcylated protein and c-Rel (75 kD) (right). The white dashed line running across all three blots indicates separation between c-Rel and p65. (E) Human PBMCs were activated with phytohemagglutinin for 48 hours. EL4 cells (50 × 106) and HEK 293T cells (10 × 106) were cultured overnight in medium containing 5 mM glucose. Cells were treated and analyzed as described in (B). (F) Jurkat cells (30 × 106) and MEFs (10 × 106) were left untreated or were treated as described in (B) and then were subjected to immunoprecipitation with succinylated WGA (sWGA) to pull down O-GlcNAcylated proteins. Samples were analyzed by Western blotting with antibodies against c-Rel and SP1, which was used as a positive control for O-GlcNAcylation and sWGA immunoprecipitation. (G) Chemoenzymatic O-GlcNAc detection assay. Jurkat cells (30 × 106) and Ramos cells (30 × 106) were left untreated or were treated with 4 mM GlcNAc and 100 µM PUGNAc for 8 hours and then were subjected to chemoenzymatic labeling and immunoprecipitation with anti–c-Rel antibody. Samples were then analyzed by Western blotting as indicated. (H) Baculoviral expression of c-Rel or p65 in insect cells in the absence or presence of OGT. Cell lysates were subjected to immunoprecipitation with antibodies against c-Rel or p65 and then were analyzed by Western blotting with an anti–O-GlcNAc antibody. Total cell lysates were analyzed by Western blotting with anti–c-Rel and anti-p65 antibodies. (I) Reverse immunoprecipitation of O-GlcNAcylated c-Rel. Jurkat cells were treated as described in (A). Anti–O-GlcNAc immunoprecipitates were analyzed by Western blotting with anti–c-Rel and anti-SP1 antibodies. Western blots in (A) through (I) are representative of three independent experiments.