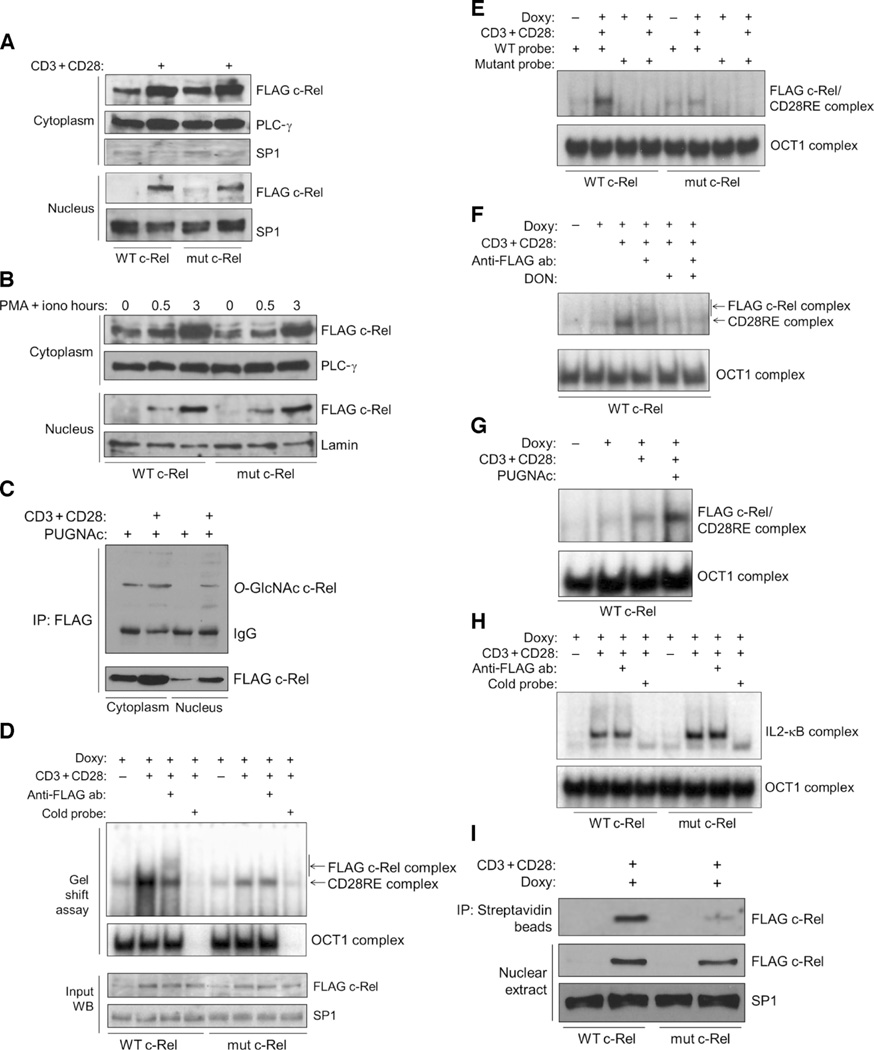
Fig. 4. Mutation of the O-GlcNAcylation site of c-Rel does not affect its nuclear translocation but impairs its binding to the CD28RE.
(A) Jurkat T-REx cell clones were treated with doxycycline for 22 hours to induce production of WT c-Rel or S350A mutant c-Rel and then were left untreated or were treated with anti-CD3 and anti-CD28 antibodies for 3 hours. Cytoplasmic and nuclear extracts were analyzed with antibodies against the indicated proteins. PLC-γ and SP1 were used as loading controls for the cytoplasmic and nuclear fractions, respectively. (B) Jurkat T-REx cell clones were treated with doxycycline for 22 hours to induce production of WT or mutant c-Rel and then were left untreated or were treated with PMA and ionomycin for the indicated times. Cytoplasmic and nuclear extracts were analyzed with antibodies against the indicated proteins. (C) Jurkat T-REx cells (75 × 106) expressing WT c-Rel were treated as described in (A). PUGNAc (100 µM) was added during the last 12 hours of doxycycline treatment. FLAG-tagged c-Rel was immunoprecipitated from cytoplasmic and nuclear extracts, and samples were analyzed by Western blotting with anti–O-GlcNAc and anti-FLAG antibodies. (D) Jurkat T-REx cell clones expressing WT c-Rel or mutant c-Rel were treated as described in (A), and nuclear extracts were analyzed by EMSA for binding to a CD28RE probe. OCT1 was used as a control for normalization of the nuclear extracts, and its specificity was determined by competition with unlabeled OCT1 probe. Bottom: Western blotting analysis of the input amounts of FLAG-tagged WT and mutant c-Rel. (E) Nuclear extracts from the samples shown in (D) were incubated with a mutated CD28RE probe as a specificity control for binding. (F) Jurkat T-REx cells expressing WT c-Rel were treated overnight with 50 µM DON. Other treatments and EMSA analyses were performed as described in (D). (G) Cells were treated overnight with 100 µM PUGNAc in culture medium containing 5 mM glucose. Other treatments and EMSA analyses were performed as described in (D). (H) Cells were treated as described in (D), and binding to the IL2-κB probe was examined by EMSA. (I) In vitro oligonucleotide binding assay. Top: Binding of WT and mutant c-Rel to the CD28RE probe. Bottom: Amounts of the respective proteins in the samples. Data in (A) to (I) are representative of three to five independent experiments. PLC-γ, lamin, and SP1 were used as loading controls.