Table 1.
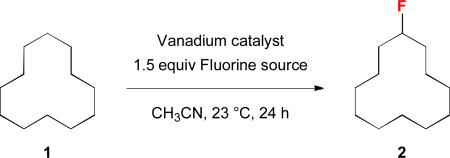
Development of the vanadium-catalyzed C—H fluorination reactiona
| ||||
|---|---|---|---|---|
| Entry | Catalyst loading | Catalyst | Fluorine source | Yieldb |
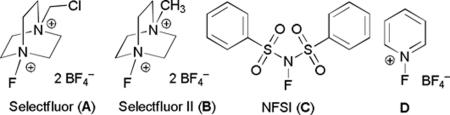
| 1 | 10 mol % | V2O5 | A | 0% |
| 2 | 20 mol % | VO(OiPr)3 | A | 0% |
| 3 | 20 mol % | VO(OSiPh3)3 | A | 0% |
| 4 | 20 mol % | VOF3 | A | 0% |
| 5 | 20 mol % | V(O)SO4 | A | 0% |
| 6 | 20 mol % | VO2 | A | <5% |
| 7 | 20 mol % | Cp2VCl2 | A | <5% |
| 8 | 20 mol % | VF3 | A | <5% |
| 9 | 20 mol % | VBr3 | A | <5% |
| 10 | 20 mol % | V(acac)3 | A | 21% |
| 11 | 10 mol % | V2O3 | A | 73% (65%)c |
| 12 | 20 mol % | Cp2V | A | 13% |
| 13 | 10 mol % | V2O3 | B | 0% |
| 14 | 10 mol % | V2O3 | C | 0% |
| 15 | 10 mol % | V2O3 | D | 0% |
| 16 | 10 mol % | – | A | 0% |
| ||||
Conditions: 1 (0.2 mmol), catalyst (0.04 mmol, or 0.02 mmol for V2O5 and V2O3), Selectfluor (0.3 mmol), CH3CN (2 mL), 23 °C.
Based on crude 19F NMR spectra using C6H5F as the external standard.
Isolated yield in parenthesis.
