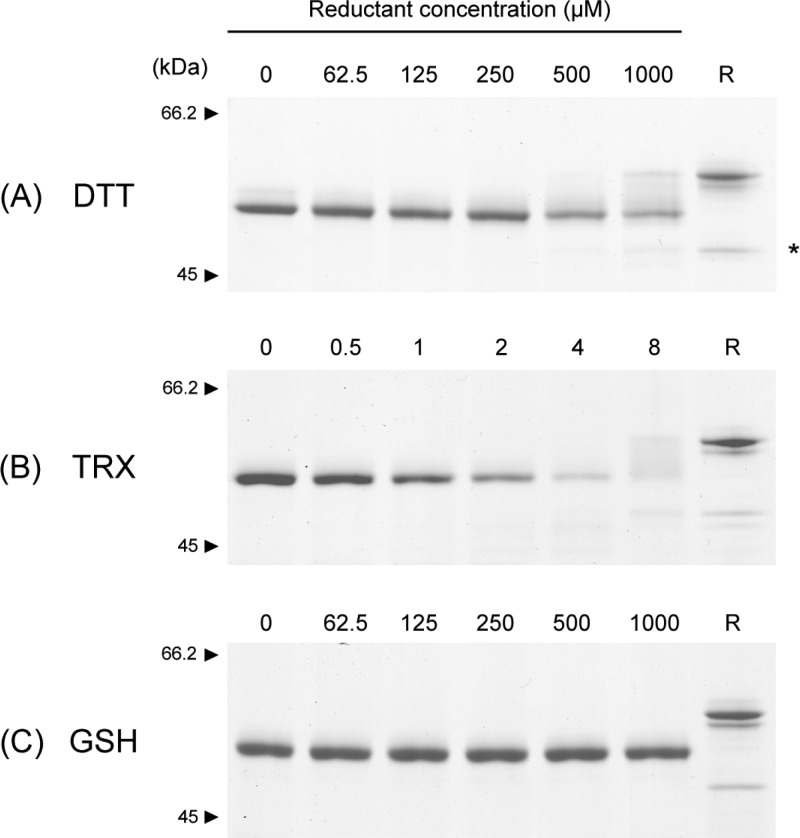
Figure 2.
Stability of HtrA1 disulfide bonds toward reducing agents. HtrA1 (1.5 μg in each lane) was incubated with freshly prepared reducing agents at the indicated concentrations for either 30 min (DTT) or 1 h (TRX and GSH) at 37 °C. Samples were then alkylated with IAA and analyzed by nonreducing SDS–PAGE using 8% gels. The reduction of HtrA1 by the different agents is reflected by the disappearance of protein from the fully oxidized band. For 4 μM TRX reduction, large cross-linked species were present at the top of the gel, representing reoxidized intermolecular disulfides and thereby explaining the apparent lack of protein at the expected sizes. A reduced control sample (R) was boiled in the presence of 5 mM DTT and subsequently alkylated with IAA before gel electrophoresis. The asterisk indicates a lower-molecular mass form of HtrA1 that is released upon reduction, representing a portion of HtrA1 that is nicked at loop residue Arg-103 or Arg-104 during cellular production and purification. The reduction of the disulfide bridges in HtrA1 is effectively achieved by DTT and TRX but not by GSH.