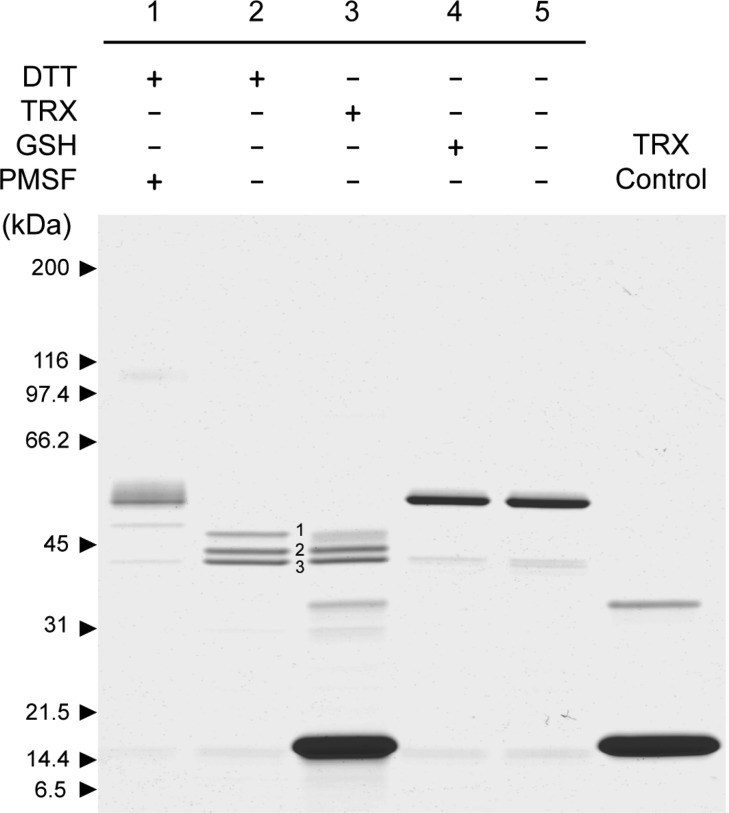
Figure 3.
Disulfide bond reduction triggers HTRA1 autolysis. To active HtrA1 (1.5 μg) was added a reducing agent (1 mM DTT, 1 mM GSH, or 4 μM TRX) and the mixture was left for 20 h at 37 °C and subsequently analyzed by nonreducing SDS–PAGE using a 5 to 15% gradient gel. Controls were included for the effect of HtrA1 activity (lane 1, PMSF-treated) and for the effect of the reducing agent (lane 5, unreduced HtrA1). TRX alone was included for comparison (lane 6). The reduction of disulfide bridges led to effective HtrA1 autolysis when proteolytic activity was not inhibited, resulting in three major autolytic products for DTT- and TRX-treated samples. The presence of a smear in lane 1 and a faint high-molecular mass band around 100 kDa (dimeric species) was caused by reoxidation of disulfide bridges by a lack of reducing power as a consequence of DTT oxidation over time.