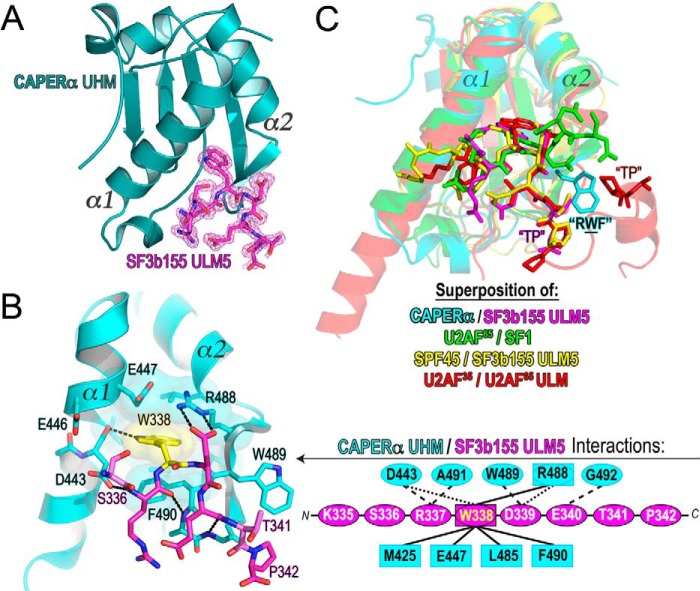
FIGURE 3.
Structure of the CAPERα UHM/SF3b155 ULM5 complex. A, structure of the CAPERα UHM (dark teal) bound to the SF3b155 ULM5 peptide. The σA-weighted |F|o − |F|c omit electron-density map for the SF3b155 ULM5 is shown in magenta at a 2.5 σ contour level. B, expanded view of the CAPERα UHM (cyan)/SF3b155 ULM5 (magenta) complex. A schematic representation of the interactions is expanded to the right. Residues involved in hydrogen bonds or salt bridges are enclosed in ovals and connected by dashed lines to represent either main-chain (long dashes) or side chain (short dashes) interactions. Residues involved in hydrophobic and aromatic interactions are enclosed in squares and connected by solid lines. C, superposition of the CAPERα UHM/SF3b155 ULM5 structure with other known UHM/ULM structures, including U2AF65 UHM/SF1 ULM (green, PDB ID 4FXW), SPF45 UHM/SF3b155 ULM5 (yellow, PDB ID 2PEH), and U2AF35 UHM/U2AF65 ULM (red, PDB ID 1JMT). Labels mark the TP motifs of the ULMs and RXF-loop of the UHM (where CAPERα Trp-489 is the representative X shown).