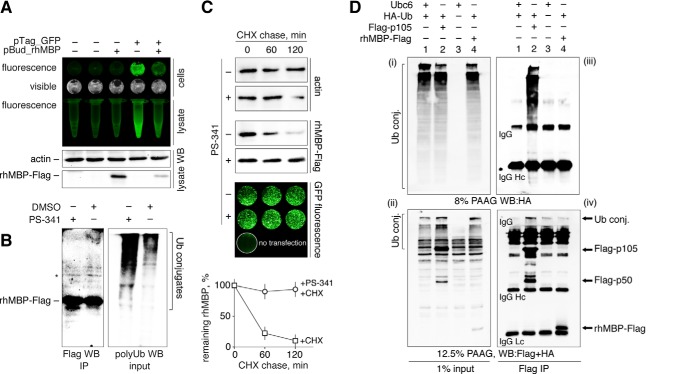
FIGURE 1.
Myelin basic protein is degraded by proteasomes in mammalian cells but shows no detectable in vivo ubiquitination. A, HEK293 cells were transfected with cDNAs coding for rhMBP and GFP. After 24 h, cells were checked for GFP fluorescence, lysed, and subjected to Western blot analysis (WB). B, HEK293 cells were transfected with cDNA coding for rhMBP-FLAG. After 24 h, cells were treated with PS-341 or dimethyl sulfoxide for 3 h, lysed, and subjected to FLAG immunoprecipitation followed by Western blot analysis. The eluates were stained for the FLAG epitope (IP, left), and the input was stained with anti-polyUb antibodies (right panel) to verify the accumulation of polyubiquitinated conjugates (conj.). An artificial band existing in all MBP samples is marked by an asterisk. C, HEK293 cells were transfected with cDNAs coding for recombinant human MBP and GFP. After 24 h, the cells were subjected to cycloheximide chase in the presence or absence of the proteasome inhibitor PS-341. The percentage of protein remaining was calculated as the ratio of protein at the time points indicated relative to the initial protein. The data are represented as the mean ± S.E. from three separate experiments. D, HEK293 cells were co-transfected with cDNAs coding for control Ubc6 without FLAG epitope and HA-Ub (1), p105-FLAG and HA-Ub (2), Ubc6 alone (3), or MBP-FLAG and HA-Ub (4). After 24 h, the cells were treated with MG132 for 2.5 h, lysed, and subjected to FLAG immunoprecipitation followed by Western blotting analysis. One percent of the total cell lysates (i–ii) and immunoprecipitates (iii–iv) were stained for HA tag (i–iii) and for HA and FLAG tag simultaneously (ii–iv). IP, immunoprecipitation; CHX, cycloheximide; PAAG, polyacrylamide gel; DMSO, dimethyl sulfoxide.