FIGURE 2.
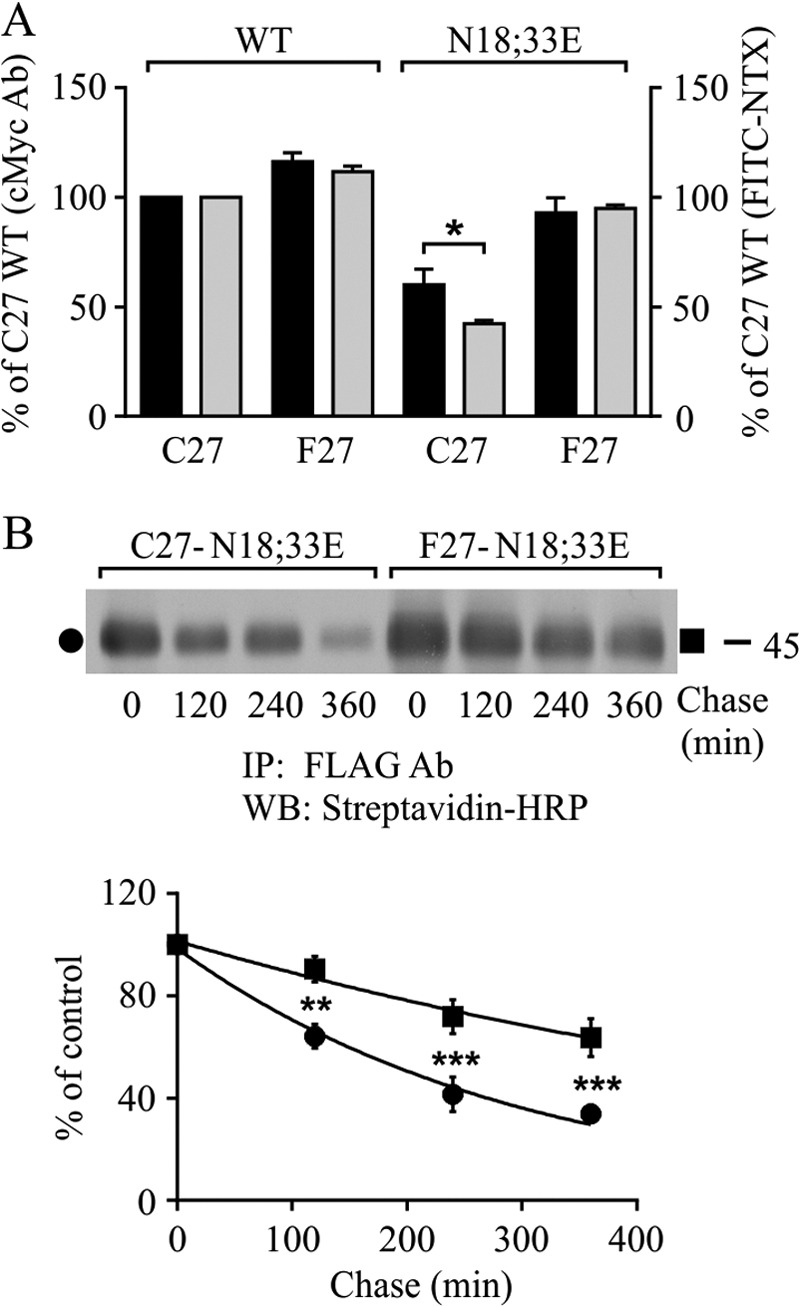
Lack of N-glycosylation destabilizes hδOR variants at the cell surface, but only the Cys27 variant shows compromised ligand binding ability. Stably transfected HEK293i cells were induced for 24 h to express WT receptor variants and their non-N-glycosylated mutants, and samples were subjected to flow cytometry (A) or cell surface biotinylation chase (B) assays. For A, cells were incubated with c-Myc (9E10) antibody followed by phycoerythrin-conjugated secondary antibody to detect the total receptor pool at the cell surface (black bars) or with the FITC-conjugated NTX (gray bars) to detect the ligand binding competent receptors. The GeoMean values were normalized to those obtained for the WT Cys27 variant. The data shown are from three to five independent experiments performed in triplicate or quadruplicate. In B, cells were labeled with sulfo-NHS-biotin and chased for the indicated periods of time at 37 °C. Receptors were immunoprecipitated from cellular lysates with FLAG M2 antibody and analyzed by Western blotting with HRP-streptavidin. The data from densitometric analysis from five independent experiments are shown. The values are means ± S.E., are normalized to the 0-h chase samples, and were fitted to the one-phase decay equation. The data were analyzed with the two-way analysis of variance and the Bonferroni's multiple comparison test. **, p < 0.01; ***, p < 0.001. Ab, antibody; IP, immunoprecipitation; WB, Western blot.
