Abstract
The ability to selectively deposit platinum black (PtB) on iridium microelectrodes and functionalize the surface for the purposes of choline sensing was investigated in this study. Platinum black was deposited by cycling 100–200 times between 0.5 V and −0.7 V in a solution of 1 mM K2PtCl6 in 0.1 M KCl. Deposition of PtB showed good chemical stability as well as good adhesion following insertion into agarose gel as a model for brain insertion. Electrode sites were also tested for their oxidative capabilities of hydrogen peroxide during which they showed high current change in response to small concentration changes - attributable to the high surface area of the PtB. Sites were then coated with an enzyme solution containing choline oxidase, and a permselective layer of meta-phenylenediamine was added to filter interferents. Electrode sites yielded a high sensitivity to choline compared to interferents including ascorbic acid and dopamine.
I. Introduction
A large part of today’s brain recording technology focuses on characterizing and studying electrical activity. The brain is often studied as an electrical circuit; however, neurotransmitters play an important role in synaptic transmission that should also be considered to better understand neurophysiology. Chemical signaling between neurons leads to excitation and inhibition of neuronal circuits and, more importantly, can help to explain neuronal plasticity [1]. The ability to record both electrical and chemical activity can lead to new insights on the interactions between neurons and can help with further developing our understanding of the structure and function of the brain.
Several technologies have been developed to monitor neurotransmitter activity of acetylcholine, dopamine, glutamate, and other neurochemicals [2–4]. Modification of multisite electrodes after fabrication, allows for a modular approach to creating probes with capabilities for sensing neurotransmitters in the brain, pH levels, and electrical activity [5, 6].
This modular functionalization of probes can be achieved using a combination of metal electrodeposition and enzyme coatings. Byproducts of the reaction of the surface enzymes with their corresponding neurochemicals can be detected via the oxidative properties of the underlying metal substrates. Past studies have used platinum sputtered sites coated with choline oxidase (ChOx) to convert choline to hydrogen peroxide (H2O2), which can then be measured through oxidation at the electrode surface [7]. Platinum acts as an excellent electrode substrate for amperometric sensing of these oxidative currents due to its inert, pseudocapacitive properties [8] and rapid electrocatalytic kinetics [9]. The aim of this study is to modify standard iridium microelectrode sites for choline sensing, through electrodeposition of platinum black (PtB), followed by application of an enzyme coating and permselective layer of meta-phenylenediamine (mPD). When deposited, PtB is porous, which can increase the effective electrode surface area and the resulting sensitivity of amperometric measurements [10, 11]. To this end, we have demonstrated and validated the deposition of PtB with a ChOx coating, towards the development of a modular probe platform with integrated chemical and electrical sensing technologies.
II. Materials & Methods
A. Silicon Probes
Probes with iridium electrode sites used in this study were supplied by the University of Michigan Center for Neural Communication Technology. Individual probes consisted of two shanks, each with two groups of tetrode sites, resulting in a total of 16 sites available for deposition per probe. Individual electrode sites were 177 μm2 in area. Iridium (Ir) electrodes are a common material used in today’s implantable neural electrodes, making them an ideal choice for post-fabrication surface modifications.
B. Electrochemical Deposition
Methods used for this study have been adopted and modified from previous studies [12, 13]. Platinum black depositions took place in a solution of 1 mM K2PtCl6 (Sigma, 206067) in 0.1 M KCl (Sigma) using potentials cycled between −0.7 V for 10 s and 0.5 V for 5 s vs. Ag|AgCl (Fisher Scientific). Each site underwent 100–200 cycles, during which platinum deposition took place at −0.7 V while the 0.5 V resting phase allowed for replenishment of platinum ions at the electrode surface. Confirmation of deposition was verified each time using a combination of visual inspection under a microscope, post-deposition impedance values, and post-deposition cyclic voltammetry (CV) measurements.
C. Electrode Characterization
Electrochemical impedance spectroscopy (EIS) and CV measurements were taken using an Autolab PGSTAT12 potentiostat (Eco Chemie, Utrecht, Netherlands). To obtain EIS and CV measurements, each probe was submerged in a phosphate buffered saline (PBS) solution of 137 mM sodium chloride, 2.7 mM potassium chloride, and 11.9 mM phosphate buffer with a large surface area platinum foil counter electrode and a standard Ag|AgCl probe as reference. Impedance measurements were taken between 10 Hz and 31 kHz at 25 mVrms. CV values were obtained by cycling from 0.8 V to −0.6 V at sweep rates of both 1 V/s and 100 mV/s.
Following deposition of PtB, each site was subjected to a 500 cycle test (CV500) of sweeping between 0.8 V and −0.6 V at a rate of 1 V/s. This range of voltages was set as the boundaries for CV cycling to ensure we did not exceed the water window [14]. To test the adherence of PtB to the underlying Ir electrode sites, after the CV500 test, each probe was inserted into 0.6% (w/v) agarose gel (Sigma) using a Precision Linear Actuator (P/N M-230.25, Polytec PI, Karlsruhe, Germany) to simulate shearing forces a probe would encounter upon true brain insertion. The probe was driven into the agarose to a depth of 2 mm at a speed of 1.2 mm/sec. After approximately five seconds, the probe was removed at the same speed and acceleration. EIS and CV measurements were taken pre-deposition, post-deposition, after CV500, and post agarose insertion/removal.
To test the oxidative capabilities of the newly formed PtB sites, probes that had not undergone CV500 or mechanical stability testing were immersed in PBS and a potential of 700 mV (vs. Ag|AgCl) was applied at each site using a BioStat System (Discovery Technology International, LLLP, Sarasota, FL). Once the baseline current values had reached steady state, increasing amounts of H2O2 in PBS were added to the initial 10 ml PBS bath to reach the following concentrations: 0.5 μM, 2.5 μM, 10 μM, 20 μM, 30 μM, and 50 μM. After each new addition of H2O2/PBS, the current was again allowed to reach steady state before more solution was added.
D. Choline Oxidase Functionalization
Probes that had been used for H2O2 sensing were then functionalized to detect choline. First a coating solution was made consisting of 1.14% (w/v) ChOx (Sigma, C5896), 0.16% (v/v) glutaraldehyde (Sigma, G5882), 1.43% (w/v) bovine serum albumin (Sigma, A9647) in PBS [15]. Sites were then coated with this mixture and refrigerated at 4 °C for at least 48 hours. A permselective layer to block interferents was then electrodeposited on the electrode sites by applying 500 mV (vs. Ag|AgCl) to individual electrode sites submersed in 0.5 mM mPD (Sigma, 78422) in PBS (purged with nitrogen gas) for 60 minutes. Probes were then calibrated for sensitivity to choline using the same concentration values as discussed above with H2O2.
III. Results
A. Impedance
Deposition of PtB is known to significantly reduce site impedance [16, 17]. Fig. 1 shows approximately an order of magnitude drop in site impedance across all tested frequencies (10 Hz to 31 kHz) after PtB deposition. Impedances rose slightly after both the CV500 and insertion tests, but stayed within close range of their lowest values achieved immediately after deposition. AC impedance serves as a helpful metric in determining platinum adherence and can be useful in other applications of PtB, including the possibility of using PtB for recording electrophysiological activity.
Figure 1.

Impedance spectra from 10 Hz – 31 kHz before and after deposition, post CV cycling, and post insertion test. Error bars represent the standard error of the mean.
B. CV Measurements
Charge storage capacity (CSC) of each site was calculated from the full area under the CV curve, scaled by the inverse of the scan rate. Initial values demonstrate the lower CSC of Ir as seen in Fig. 2, where values are less than 1000 μC/cm2. Deposition of PtB greatly increased CSC by a factor of over one order of magnitude, pointing to the large increase in surface area due to the rough PtB surface [10]. Values acquired after CV500 and post-insertion show a drop; however, values are still at least one order of magnitude larger than initial values for both sweep rates.
Figure 2.

Average CSC values for each sweep rate taken before and after deposition, after CV500, and after mechanical insertion. Error bars represent standard error of the mean.
To characterize the electrochemical stability of the PtB deposition, each site was also cycled between −0.6 V and 0.8 V, 500 times. Fig. 3 depicts the multiple CV plots for a representative site whose peaks and dips match those of previous findings [14]. After a short time period, cycles begin to quickly overlap indicating a brief change in performance, followed by electrochemical stability. This behavior corresponds well with Fig. 3 where the average CSC values for all sites demonstrate a rapid increase and then quickly level off to more stable values.
Figure 3.

CV500 Test Results. (Top) Initial CV cycles are in blue and progress to warmer colors as more cycles take place. As the colors progress from cold to warm less variation is present and plots largely overlap one another. (Bottom) Average charge storage capacity of electrode sites during the CV500 test. Multiple sites across one probe rapidly reach a maximum value and hold steady over cycles, where the error bars represent the standard error of the mean.
C. Hydrogen Peroxide Oxidation & Choline Sensing
Sensitivity values reflect the capability of electrode sites to detect varying concentrations of either H2O2 or choline. Larger values indicate that a given site performs better at sensing small concentration changes of a given chemical. The mechanism of detection starts with the oxidation of H2O2 at the PtB surface. The resulting oxidation causes a change in the baseline current, which is then used as a measure of analyte concentration. Measuring choline is also achieved through the oxidation of H2O2, one of two byproducts released in the enzymatic breakdown of choline by choline oxidase [18].
Multiple H2O2 concentration sensitivity studies were conducted, resulting in average site-specific sensitivity values (Fig. 4). This metric was used to demonstrate that the uncoated PtB maintained stable oxidative performance over multiple experiments. Sites coated with the enzyme solution and mPD were then characterized in response to increasing concentrations of choline (Fig. 4). The observed lower choline sensitivity values in comparison to H2O2, corresponds with established literature reflecting the decreased choline accessibility to surface ChOx by the mPD coating and subsequently the ability of the PtB to respond to the resultant H2O2 [19]. Sites also maintained good selectivity over potential interferents including ascorbic acid (AA) and dopamine (DA) (data not shown).
Figure 4.
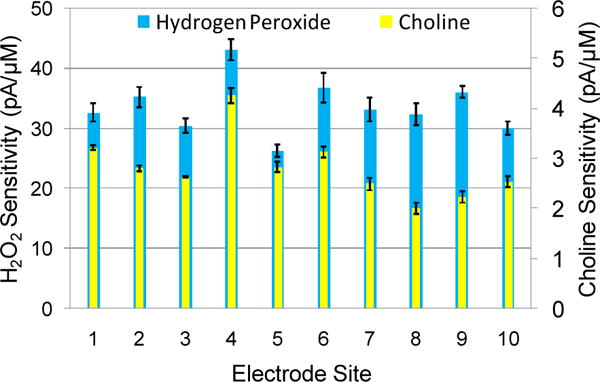
H2O2 and choline sensitivity for PtB microelectrode sites. Each site underwent three measurement trials with the average value being displayed with standard error bars of the mean.
IV. Discussion
The purpose of this study was to demonstrate the feasibility of functionalizing iridium multi-site microelectrodes with platinum black as a surface substrate for detecting choline, using an overlaying coating of choline oxidase and a permselective mPD coating. Our findings here have shown that PtB is a suitable material for choline sensing and additionally has electrical and mechanical qualities that would make it a good candidate for use in electrophysiological recordings and electrical stimulation applications.
Results from both EIS and CV measurements have confirmed both the adherence of the PtB and increased surface area while still maintaining a small footprint. Past literature has commented on the poor adherence of electrodeposited PtB [11]. After insertion into an agar model of brain tissue, PtB sites showed some deformities, but overall quality of the deposition was not severely compromised as indicated through impedance and CV measurements. While not examined in this study, it is likely that the enzyme functionalization and permselective coatings may protect the PtB from shear forces. Impedance values (Fig. 1) may have risen after both CV500 and mechanical insertion, yet they still remain in a range sufficient to maintain function. The overall magnitude increase in CSC underscores the large area of PtB available to store charge and more importantly, to serve as a reactive surface for the oxidation of H2O2, without damaging or degrading the electrode surface. However, the drop in the CSC observed following the post-CV500 or mechanical insertion tests could be accounted for by the loss of loose platinum at the site surface or deformation that sites may undergo upon insertion.
PtB microelectrode sites show significant potential for in vivo biosensing of neurotransmitter release. PtB sites demonstrated sensitivity to H2O2 and choline. Choline sensitivity was approximately one order of magnitude lower than that of H2O2, most likely due to the permselective coating [19]. This result is expected, as not all H2O2 molecules created by ChOx will diffuse to the electrode surface. The highly porous nature of PtB provides increased surface area within the same geometric area as sputtered electrode sites and thus can provide increased sensitivity. In addition, the sensing capabilities of the probe were able to distinguish choline over the interferents of AA and DA. Further optimization of the enzyme and mPD coating procedures specific to the PtB electrodes may improve choline sensitivity, limits of detection, and selectivity over potential interferents.
Acknowledgments
The authors would like to thank Dr. Daryl Kipke for paper comments, Rachel Miriani for help with experimental planning, and the rest of the Neural Engineering Lab at the University of Michigan.
This work was supported by the Center for Neural Communication Technology - a P41 Resource Center funded by the National Institute of Biomedical Imaging and Bioengineering (NIBIB, P41 EB002030) and supported by the National Institutes of Health (NIH).
Contributor Information
Paras R. Patel, Email: parasp@umich.edu, Department of Biomedical Engineering, University of Michigan, Ann Arbor, MI 48109 USA.
Matthew D. Gibson, Email: mattgib@umich.edu, Center for Entrepreneurship, University of Michigan, Ann Arbor, MI 48109 USA
Kip A. Ludwig, Email: kip.ludwig@nih.gov, Department of Biomedical Engineering, University of Michigan, Ann Arbor, MI 48109 USA
Nicholas B. Langhals, Email: langhals@umich.edu, Department of Biomedical Engineering & Section of Plastic Surgery, University of Michigan, Ann Arbor, MI 48109 USA.
References
- 1.Flagel SB, Clark JJ, Robinson TE, Mayo L, Czuj A, Willuhn I, Akers CA, Clinton SM, Phillips PEM, Akil H. A selective role for dopamine in stimulus-reward learning. Nature. 2011 Jan;469(7328):53–57. doi: 10.1038/nature09588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Frey O, van der Wal PD, Spieth S, Brett O, Seidl K, Paul O, Ruther P, Zengerle R, de Rooij NF. Biosensor microprobes with integrated microfluidic channels for bi-directional neurochemical interaction. J Neural Eng. 2011 Dec;8(6):066001. doi: 10.1088/1741-2560/8/6/066001. [DOI] [PubMed] [Google Scholar]
- 3.Quintero JE, Pomerleau F, Huettl P, Johnson KW, Offord J, Gerhardt GA. Methodology for rapid measures of glutamate release in rat brain slices using ceramic-based microelectrode arrays: Basic characterization and drug pharmacology. Brain Res. 2011 Jul;1401:1–9. doi: 10.1016/j.brainres.2011.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Parikh V, Salter M. Cortical choline transporter function measured in vivo using choline-sensitive microelectrodes: clearance of endogenous and exogenous choline and effects of removal of cholinergic terminals. J Neurochem. 2006 Apr;97(2):488–503. doi: 10.1111/j.1471-4159.2006.03766.x. [DOI] [PubMed] [Google Scholar]
- 5.Johnson MD, Franklin RK, Gibson MD, Brown RB, Kipke DR. Implantable microelectrode arrays for simultaneous electrophysiological and neurochemical recordings. J Neurosci Methods. 2008 Sep;174(1):62–70. doi: 10.1016/j.jneumeth.2008.06.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Johnson MD, Kao OE, Kipke DR. Spatiotemporal pH dynamics following insertion of neural microelectrode arrays. J Neurosci Methods. 2007 Mar;160(2):276–287. doi: 10.1016/j.jneumeth.2006.09.023. [DOI] [PubMed] [Google Scholar]
- 7.Cremisini C, Sario SD, Mela J, Pilloton R, Palleschi G. Evaluation of the use of free and immobilised acetylcholinesterase for paraoxon detection with an amperometric choline oxidase based biosensor. Anal Chim Acta. 1995 Aug;311(3):273–280. [Google Scholar]
- 8.Whalen JJ, III, Young J, Weiland JD, Searson PC. Electrochemical Characterization of Charge Injection at Electrodeposited Platinum Electrodes in Phosphate Buffered Saline. J Electrochem Soc. 2006;153(12):C834–C839. [Google Scholar]
- 9.Burmeister JJ, Palmer M, Gerhardt GA. Ceramic-based multisite microelectrode array for rapid choline measures in brain tissue. Anal Chim Acta. 2003 Mar;481(1):65–74. [Google Scholar]
- 10.Whalen JJ, III, Weiland JD, Searson PC. Electrochemical Deposition of Platinum from Aqueous Ammonium Hexachloroplatinate Solution. J Electrochem Soc. 2005;152(11):C738–C743. [Google Scholar]
- 11.Ilic B, Czaplewski D, Neuzil P, Stanczyk T, Blough J, Maclay GJ. Preparation and characterization of platinum black electrodes. J Mater Sci. 2000;35(14):3447–3457. [Google Scholar]
- 12.Dai X, Compton RG. Detection of As(III) via oxidation to As(V) using platinum nanoparticle modified glassy carbon electrodes: arsenic detection without interference from copper. Analyst. 2006 Apr;131(4):516–521. doi: 10.1039/b513686e. [DOI] [PubMed] [Google Scholar]
- 13.Cui HF, Ye JS, Zhang WD, Wang J, Sheu FS. Electrocatalytic reduction of oxygen by a platinum nanoparticle/carbon nanotube composite electrode. J Electroanal Chem. 2005 Apr;577(2):295–302. [Google Scholar]
- 14.Cogan SF. Neural stimulation and recording electrodes. Annu Rev Biomed Eng. 2008;10:275–309. doi: 10.1146/annurev.bioeng.10.061807.160518. [DOI] [PubMed] [Google Scholar]
- 15.Parikh V, Pomerleau F, Huettl P, Gerhardt GA, Salter M, Bruno JP. Rapid assessment of in vivo cholinergic transmission by amperometric detection of changes in extracellular choline levels. Eur J Neurosci. 2004 Sep;20(6):1545–1554. doi: 10.1111/j.1460-9568.2004.03614.x. [DOI] [PubMed] [Google Scholar]
- 16.Desai SA, Rolston JD, Guo L, Potter SM. Improving impedance of implantable microwire multi-electrode arrays by ultrasonic electroplating of durable platinum black. Front Neuroeng. 2010 May;3 doi: 10.3389/fneng.2010.00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Paik SJ, Park Y, Cho D. Roughened polysilicon for low impedance microelectrodes in neural probes. J Micromech Microeng. 2003 May;13(3):373–379. [Google Scholar]
- 18.Damsma G, van Bueren DL, Westerink BHC, Horn AS. Determination Of Acetylcholine And Choline In The Femtomole Range By Means Of Hplc, A Post-Column Enzyme Reactor, And Electrochemical Detection. Chromatographia. 1987 Dec;24(1):827–831. [Google Scholar]
- 19.Chen X, Matsumoto N, Hu Y, Wilson GS. Electrochemically mediated electrodeposition/electropolymerization to yield a glucose microbiosensor with improved characteristics. Anal Chem. 2002 Jan;74(2):368–372. doi: 10.1021/ac015628m. [DOI] [PubMed] [Google Scholar]


