Fig. 2.
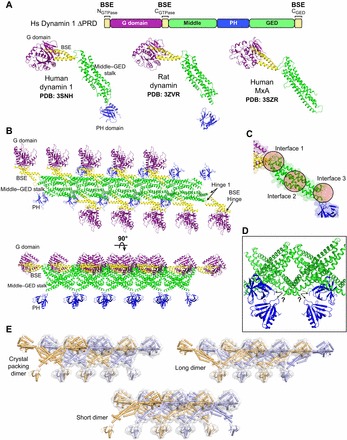
Structures and crystal packing of dynamin family members. (A) X-ray structures of intact human dynamin 1, rat dynamin and human MxA. Each protein contains different mutations that yield stable dimers in solution. Only the monomers are shown. The linear domain arrangement of human dynamin 1 is shown above for comparison. Coloring is as follows: G domain, purple; BSE, yellow; middle–GED stalk, green; PH domain, blue. (B) Dynamin-related proteins crystallize in linear arrays that are mediated primarily by stalk–stalk interactions. Rat dynamin crystal packing (PDB: 3ZVR) is depicted in two orientations (top view above, side view below). (C) Magnified view of the dynamin stalk illustrating the three interfaces (circles) that mediate intermolecular interactions within the crystallized linear dynmain array. Mutations that result in the monodisperse dimers used for crystallization map to interface 3. (D) Disordered regions at the base of the stalk yield an ambiguity (black arrows) in determining the stalk–PH connectivity. (E) Different putative domain connectivities within the mutant dynamin dimers can conform to the same crystal packing. Alternate dimers are colored orange and light blue. The light gray surface illustrates how different arrangements all yield the same overall shape and packing.
