Fig. 1.
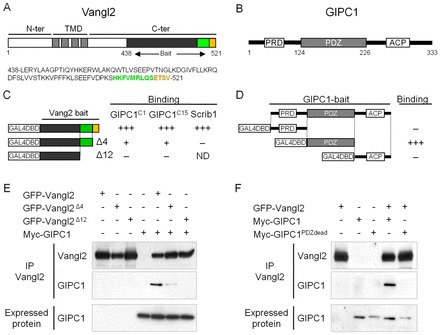
Gipc1 is a binding partner for Vangl2. (A) Vangl2 is a four transmembrane-domain (TMD) protein, with intracellular N-terminus (N-ter) and C-terminus (C-ter) ending with a PDZ binding motif (-ETSV, orange). The bait used to screen the embryonic cochlear library is indicated (aa 438-521). (B) Gipc1 is a cytosolic protein of 333 aa with a central PDZ domain, an N-ter proline-rich domain (PRD) and a C-ter acyl carrier protein domain (ACP). (C,D) Yeast-two-hybrid assay validating the interaction between Gipc1 and Vangl2. (C) The bait of Vangl2 positively interacts with two of the clones (GIPC1C1 and GIPC1C15) from the yeast-two-hybrid screen. Deletion of the PDZ-BM (last four aa, Δ4) leads to a strong reduction of the interaction, which is completely inhibited by deletion of the last 12 aa of the C-ter (Δ12). The interaction with Scrib1 was used as a positive control. (D) Only the PDZ domain of Gipc1 interacts with Vangl2. (E,F) Co-immunoprecipitation (co-IP) of GFP-Vangl2 with myc-Gipc1. (E) The interaction is strongly impaired by deletion of the PDZ-BM and abolished by removal of the last 12 aa of Vangl2. (F) The interaction is disrupted by mutation of the PDZ domain of Gipc1.
