Abstract
Background
Recent studies have shown that influenza is associated with significant disease burden in many countries in the tropics, but until recently national surveillance for influenza was not conducted in most countries in Africa.
Methods
In 2007, the Kenyan Ministry of Health with technical support from the CDC-Kenya established a national sentinel surveillance system for influenza. At 11 hospitals, for every hospitalized patient with severe acute respiratory illness (SARI), and for the first three outpatients with influenza-like illness (ILI) per day, we collected both nasopharyngeal and oropharyngeal swabs. Beginning in 2008, we conducted in-hospital follow-up for SARI patients to determine outcome. Specimens were tested by real time RT-PCR for influenza A and B. Influenza A-positive specimens were subtyped for H1, H3, H5, and (beginning in May 2009) A(H1N1)pdm09.
Results
From July 1, 2007 through June 30, 2013, we collected specimens from 24,762 SARI and 14,013 ILI patients. For SARI and ILI case-patients, the median ages were 12 months and 16 months, respectively, and 44% and 47% were female. In all, 2,378 (9.6%) SARI cases and 2,041 (14.6%) ILI cases were positive for influenza viruses. Most influenza-associated SARI cases (58.6%) were in children <2 years old. Of all influenza-positive specimens, 78% were influenza A, 21% were influenza B, and 1% were influenza A/B coinfections. Influenza circulated in every month. In four of the six years influenza activity peaked during July–November. Of 9,419 SARI patients, 2.7% died; the median length of hospitalization was 4 days.
Conclusions
During six years of surveillance in Kenya, influenza was associated with nearly 10 percent of hospitalized SARI cases and one-sixth of outpatient ILI cases. Most influenza-associated SARI and ILI cases were in children <2 years old; interventions to reduce the burden of influenza, such as vaccine, could consider young children as a priority group.
Introduction
Influenza causes substantial morbidity and mortality worldwide every year. In 2008, in children <5 years old, an estimated 90 million new cases of influenza occurred, including an estimated 28,000–111,500 deaths, nearly all of which (99%) occurred in developing countries [1]. While seasonal influenza has long been recognized as a cause of morbidity and mortality in developed countries with temperate climates, recent studies have shown that influenza causes a significant burden of disease in countries throughout the tropics as well [2]–[4].
In Sub-Saharan Africa, surveillance for influenza has been extremely limited, likely due to a combination of limited public health infrastructure in the region, and competing health priorities such as HIV, tuberculosis, and malaria [5]–[9]. However, in 2005, in response to the threat of avian influenza A(H5N1), governments throughout the world and international organizations such as the World Health Organization (WHO) began investing more resources in influenza pandemic preparedness. As a result, a number of countries in Africa were able to use public health resources to improve their capacity to conduct virological and epidemiological surveillance for influenza [10], [11].
In Kenya, an equatorial country in East Africa with a mostly tropical climate, few data are available on influenza. In 2006, the Kenyan Ministry of Health, with technical support from the Centers for Disease Control and Prevention-Kenya (CDC-K), established a national sentinel surveillance system for influenza. The objectives of the surveillance system were to identify circulating influenza strains, to understand the epidemiology and burden of influenza in Kenya, and to serve as a component of an early warning system for pandemic influenza.
Methods
Study sites
Beginning in August 2006, The Kenyan Ministry of Health (MOH) and CDC-K initiated influenza surveillance at a national referral hospital (Kenyatta National Hospital) in Nairobi, seven provincial general hospitals (Coast, Embu, Garissa, Kakamega, Nakuru, New Nyanza and Nyeri), and two refugee camps (Kakuma and Dadaab) (Figure 1). At each site, surveillance was conducted for influenza-like illness (ILI) and severe acute respiratory illness (SARI). In August 2009, in order to integrate influenza surveillance with other ongoing surveillance activities in Nyanza Province, we initiated SARI surveillance at Siaya District Hospital (SDH) and ILI surveillance at Tingwang'i Health Center (THC), and discontinued influenza surveillance at New Nyanza Provincial Hospital.
Figure 1. Map of Kenya showing the distribution of influenza sentinel surveillance site.
A trained surveillance officer – either a nurse or a clinical officer – was employed at each of the sites. Officers identified ILI patients at outpatient clinics and emergency rooms, and SARI patients in adult and pediatric in-patient wards in the hospitals. From August 2006 through December 2011, for every consented person with SARI and for the first three consented ILI patients per weekday at each site, a two-page questionnaire with questions about demographics, underlying diseases, influenza vaccine history, signs and symptoms, and exposures, was administered, and a combined nasopharyngeal (NP) swab and oropharyngeal (OP) swab was collected. From January 2012–June 2013, surveillance was conducted at nine hospitals only (surveillance at Embu and Garissa was discontinued) and was limited to SARI in all but 2 locations (Kenyatta National Hospital and Tingwan'I Health Center). Beginning in 2008, we conducted in-hospital follow-up for SARI patients to determine final outcome (discharge or death). Deaths that occurred within 30 days of hospital admission were considered to be associated with SARI.
Case Definitions
The following case definitions were used:
Influenza-Like Illness
Influenza-like illness was defined as an axillary temperature ≥38°C and cough or sore throat in an outpatient of any age.
Severe Acute Respiratory Illness
The case definition used for severe acute respiratory illness was different for persons <5 and those ≥5 years old. For children <5 years old, a modified version of the World Health Organization's Integrated Management of Childhood Illness (IMCI) definition for pneumonia [12] was used, requiring the presence of cough or difficulty breathing plus one of the following danger signs: chest in-drawing, stridor, unable to breastfeed or drink, vomits everything, convulsions, lethargy, or unconsciousness. For patients ≥5 years old, SARI was defined as axillary temperature ≥38°C plus cough, difficulty breathing or shortness of breath. Hospitalization was a required criterion for SARI cases, regardless of age.
Specimen collection
For each patient, NP and OP swabs were placed into a single cryovial with viral transport medium (VTM), which was prepared at the Kenya Medical Research Institute(KEMRI)/CDC laboratory in Nairobi according to a WHO protocol [13] and routinely distributed to each of the surveillance sites. The procedure for specimen collection has been previously described [14]. Specimens were immediately refrigerated at 2–8 degrees Celsius. Specimens from all sites underwent triple packaging, and were transported by courier service or hand-carried to the National Influenza Center (NIC) in Nairobi, where they were immediately placed in a −80°Celsius freezer. Samples from the two refugee camps were transported directly to the KEMRI/CDC-K laboratory in Nairobi by scheduled flights.
Laboratory Testing
At the NIC and the KEMRI/CDC laboratories, specimens were tested by real time reverse transcription-polymerase chain reaction (rRT-PCR) for influenza A and influenza B viruses. Total ribonucleic acid (RNA) was extracted from 140 µL aliquots of each specimen using a QIAamp viral RNA mini kit (Qiagen GmbH, Germany) according to the manufacturer's instructions. One step rRT-PCR was carried out using the AgPath kit (Applied Biosystems, California, USA). Following the reverse transcription step, a typical 45 cycle PCR reaction was run and fluorescence was read at the annealing/extension step. Appropriate negative and positive control specimens were run alongside each reaction. The results were recorded as cyclic threshold (CT) values. When all controls met the stated requirements, any influenza A and B CT value <40 was recorded as positive. Values with a CT reading ≥40 were recorded as negative.
All specimens positive for influenza A were sub-typed for H1, H3, H5, and (beginning in May 2009) pH1N1 using rRT-PCR. Specimens that were positive for influenza A virus by rRT-PCR but failed to sub-type were sent to the WHO Influenza Collaborating Center at CDC-Atlanta, GA, USA for further antigenic characterization.
Data Management
Data from all but two sites were collected using standard paper questionnaires and entered into a Microsoft Access database. At Siaya District Hospital and Tingwang'i Health Center, scannable paper forms were used (TeleForm software, Cardiff, Vista, CA). Beginning in May 2011 smart phones (HTC Touch Pro 2 model) were used to collect the same demographic and clinical data from patients in five hospitals. Data collected on smart phones were uploaded daily to the KEMRI/CDC server in Kisumu using a secure web link. Data were downloaded from the server onto an MS Access database. We conducted data cleaning to check for inconsistent or illogical data every week. Laboratory data were merged with epidemiologic data weekly.
Data Analysis
Data from July 2007, the first month when surveillance was conducted in all 11 sites, through June 2013, were included in the analysis. We excluded patients for whom demographic data or laboratory results were missing, and patients who did not meet one of the case definitions. We used Pearson's chi square test to compare demographic and clinical variables between SARI and ILI patients. We used logistic regression to calculate odds ratios in bi-variate and multivariate analyses comparing influenza-positive and influenza-negative ILI patients, and influenza-positive and influenza-negative SARI patients. We used generalized linear models (GLM) to estimate relative risk when comparing fatal and non-fatal cases. Multivariable logistic regression and GLM models were constructed using factors that were significant at p<0.2 in the bi-variate analysis. SARI patients <5 years old and those ≥5 years old were analyzed separately because different case definitions were used for the two age groups. Findings were considered statistically significant if the p-value was <0.05. Data analyses were performed using Stata 12.1 (Stata Corp. 2011. Stata Statistical Software: Release 12. College Station, TX: Stata Corp LP).
Ethical considerations
The Kenya Ministry of Health (KMoH) issued a document stating that sentinel surveillance for influenza, including follow-up in-hospital surveillance, should be considered part of routine public health surveillance, and therefore did not require formal ethical review. Because the activity was classified as routine surveillance, the KMoH considered verbal consent to be adequate. Verbal consent was obtained from all patients before questionnaires were administered and specimens were collected. For children, verbal consent was obtained from guardians. Surveillance officers documented consenting and non-consenting participants in a log book at each site. Authors did not participate in sample collection. Data was anonymized upon collection, and authors did not have access to identifying information.
Results
From July 1, 2007 through June 30, 2013, a total of 38,775 (24,762 SARI and 14,013 ILI) specimens were collected from patients in the 11 sentinel surveillance sites. Overall, 90% of SARI samples and 87% of ILI samples were collected from children <5 years (Table 1). The median ages of SARI and ILI cases were 1 year (range: 1 month–95 years) and 1 year and 4 months (range: 1 month–75 years), respectively. Of the SARI and ILI cases, 10,823 (44%) and 6,582 (47%) were female, respectively. A significantly greater percentage of SARI patients reported having any underlying and chronic conditions compared to ILI patients (6.8% vs. 2.3%, p<0.001). The most common underlying medical conditions and chronic symptoms reported were recurrent chest pain (2.0%) and asthma (1.5%) among SARI patients, and asthma (0.6%) and chronic shortness of breath (0.5%) among ILI patients. The median duration of illness from the date of symptom onset to presentation at the health facility was three days [interquartile range (IQR): 2–5 days] for SARI patients, and 2 days (IQR: 1–3 days) for ILI patients. Although overall 1.5% of all SARI and ILI patients reported having received influenza vaccination in the previous year, the majority of these patients (60%) were from Kakuma Refugee Camp (data not shown), where free seasonal influenza vaccine was distributed by the Kenyan Ministry of Public Health and Sanitation in 2010. Overall 16.0% of SARI patients <5 years old, 18.6% of SARI patients ≥5 years old and 9.7% of all ILI patients had been hospitalized during the previous year.
Table 1. Characteristics of patients and comparison of SARI and ILI, Kenya, July 2007–June 2013.
| SARI (N = 24,762) | ILI (N = 14,013) | ||
| n (%) | n (%) | p-value | |
| Sex | |||
| Female | 10823(43.7) | 6582(47.0) | <0.001 |
| Age Group | |||
| 0–23 months | 16709(67.5) | 7916(56.5) | <0.001 |
| 24–59 months | 5632(22.7) | 4281(30.6) | |
| 5–9 years | 1247(5.0) | 1199(8.6) | |
| 10–17 years | 360(1.5) | 269(1.9) | |
| 18–49 years | 684(2.8) | 321(2.3) | |
| ≥50 years | 130(0.5) | 27(0.2) | |
| Mean age years (SD) | 2.8(6.9) | 2.9(5.2) | |
| Median age years (IQR) | 1.0(0.5–2.0) | 1.3(0.8–3.0) | |
| Site | |||
| Kenyatta NH | 2138(8.6) | 1443(10.3) | <0.001 |
| Coast PGH | 2112(8.5) | 809(5.8) | |
| Nakuru PGH | 2094(8.5) | 1869(13.3) | |
| Nyeri PGH | 3118(12.6) | 1999(14.3) | |
| Kakamega PGH | 4300(17.4) | 2022(14.4) | |
| Embu PGH | 430(1.7) | 794(5.7) | |
| Garissa PGH | 764(3.1) | 546(3.9) | |
| Kakuma | 3705(15.0) | 1477(10.5) | |
| Dadaab | 2880(11.6) | 1303(9.3) | |
| Siaya DH | 3125(12.6) | 0(0.0) | |
| Ting'wang'i HC | 0(0.0) | 1649(11.8) | |
| Nyanza PGH | 96(0.4) | 102(0.7) | |
| Length of illness before presenting to the hospital | |||
| 0–3 days | 14299(57.7) | 10588(75.6) | <0.001 |
| 4–7 days | 8959(36.2) | 3037(21.7) | |
| >7 days | 1504(6.1) | 388(2.8) | |
| Mean duration of illness (SD) | 3.7(2.8) | 2.6(2.5) | |
| Median duartion of illness (IQR) | 3.0(2.0–5.0) | 2.0(1.0–3.0) | |
| Underlying chronic illnesses a | |||
| Any chronic illness | 1695(6.8) | 327(2.3) | <0.001 |
| Heart disease | 114(0.5) | 25(0.2) | <0.001 |
| Chronic shortness of breath | 269(1.1) | 70(0.5) | <0.001 |
| Recurrent chest pain | 507(2.0) | 16(0.1) | <0.001 |
| Asthma | 370(1.5) | 85(0.6) | <0.001 |
| Chronic cough (≥3 months in 2 consecutive years) | 144(0.6) | 58(0.4) | 0.021 |
| Active TB | 147(0.6) | 19(0.1) | <0.001 |
| Reported vaccination against influenza in the past year | 375(1.5) | 222(1.6) | 0.943 |
| Influenza Viruses | |||
| Influenza A and/or B | 2378(9.6) | 2041(14.6) | <0.001 |
| 2007 | 250/2,245(11.1) | 238/1,505(15.8) | |
| 2008 | 354/4,106(8.6) | 378/2,883(13.1) | |
| 2009 | 504/4,200(12.0) | 609/3,351(18.2) | |
| 2010 | 458/4,775(9.6) | 421/3,361(12.5) | |
| 2011 | 503/5,593(9.0) | 343/2,224(15.4) | |
| 2012 | 199/2,758(7.2) | 39/479(8.1) | |
| 2013 | 110/1,085(10.1) | 13/210(6.2) | |
| Influenza A | 1853(7.5) | 1621(11.6) | <0.001 |
| Pandemic H1N1 * | 421/1,313 (32.1) | 391/1,054(37.1) | |
| Seasonal A (H1N1) * | 138/1,853(7.4) | 218/1,621(13.4) | |
| Seasonal A (H3N2) * | 478/1,853(25.8) | 407/1,621(25.1) | |
| Unsubtyped * b | 0/1,853(0.0) | 4/1,621(0.2) | |
| Not subtyped * c | 817/1,853(44.1) | 603/1,621(37.2) | |
| Influenza B | 545(2.2) | 462(3.3) | <0.001 |
| Influenza A and B co-infection | 20(0.1) | 42(0.3) | <0.001 |
These data were not collected in Siaya DH and Tingwangi.
Flu A positive samples with CT values ≤37 which could not be subtyped.
Flu A positive samples that were not subtyped. Most of these (95%) were samples tested before June 2008 when subtyping started.
*Denominator = Total Influenza A Cases.
In all, 2,378 (9.6% of total) SARI cases and 2,041 (14.6%) of ILI cases were positive for influenza viruses (p<0.001, Table 1). For years in which a complete calendar year of surveillance was conducted, the percentage of influenza-positive SARI cases ranged from a low of 7.2% in 2012 to a high of 12.0% in 2009, while the percentage of influenza-positive ILI cases ranged from a low of 8.1% in 2012 to a high of 18.2% in 2009. Of all influenza-positive SARI specimens, 77.1% were influenza A, 22.1% were influenza B, and 0.8% were co-infections with influenza A and B. Among the influenza-positive ILI samples collected, 77.2% were influenza A, 20.7% were influenza B, and 2.1% were co-infections. Most influenza A samples that were subtyped were either H3 (25.8% and 25.1% of SARI and ILI patients, respectively) or A(H1N1) pdm09 (32.1% and 37.1% of SARI and ILI patients tested after May 2009, respectively).
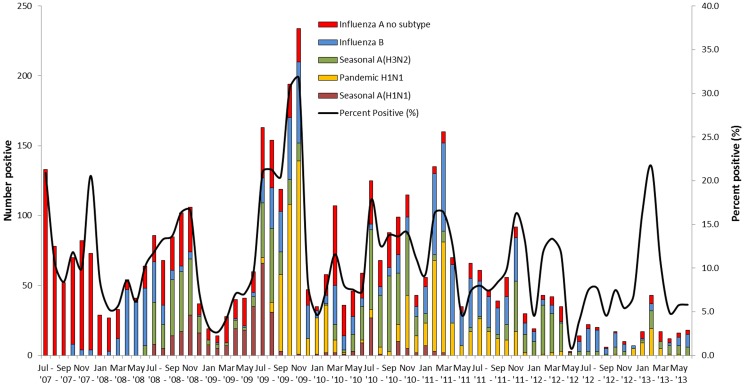
In the six years of surveillance, influenza circulated nearly every month. There were periods of increased influenza activity, particularly during the initial months that pandemic influenza A (H1N1) virus circulated in Kenya in late 2009. In four of the six years (2008–2011) the highest absolute number of influenza-positive samples and the highest percentage-positive of SARI specimens for influenza occurred during July to November (Figure 2). Influenza B and influenza A (H3N2) viruses circulated throughout the six years. Influenza A (H1N1)pdm 09 virus circulated consistently after its first observation in July 2009, while seasonal H1N1 virus was prominent in 2008 and 2009, and appeared sporadically in 2010.
Figure 2. Seasonality of influenza for ILI and SARI patients, Kenya, July 2007–June 2013.
Note: Smoothing done using the double exponential smoothing methods.
Among SARI patients <5 years old, there was a higher percentage of influenza positivity in children 24–59 months old compared to patients ≤23 months old [adjusted Odds Ratio (aOR) = 1.3, 95% Confidence Interval (CI) = 1.2–1.5] (Table 2). Asthma was less common in influenza-positive children compared to influenza-negative children (aOR = 0.5, 95% CI = 0.3–0.9). Fever and convulsions were slightly more common in influenza-positive patients, while weight loss was more common in influenza-negative children. Among SARI patients ≥5 years old, no demographic factors were associated with influenza cases, and headache was the only symptom more common in influenza-positive cases compared with influenza-negative cases (aOR = 1.4 (95% CI = 1.1–2.0) (Table 3). For ILI and SARI patients <5 years old and ≥5 years old, in multivariate analysis, the percentage of influenza positivity varied by hospital (p<0.001 for all three comparisons).
Table 2. Laboratory-confirmed influenza infection among SARI patients aged <5 years, by demographic and clinical characteristics, at 9 sites in Kenya, July 2007–June 2013.
| Total (N = 22,341) | Influenza positive patients (N = 2,021) | Influenza negative patients (N = 20,320) | Crude OR (95% CI) | p-Value | Adjusteda OR (95% CI) | p-Value | |
| n(%)¥ | n(%)¥ | ||||||
| Sex | |||||||
| Male | 12,644 | 1170(9.3) | 11474(90.7) | Ref | |||
| Female | 9,697 | 851(8.8) | 8846(91.2) | 0.9(0.9–1.0) | 0.218 | - | - |
| Age Group | |||||||
| 0–23 months | 16,709 | 1395(8.3) | 15314(91.7) | Ref | Ref | ||
| 24–59 months | 5,632 | 626(11.1) | 5006(88.9) | 1.4(1.2–1.5) | <0.001 | 1.3(1.2–1.5) | <0.001 |
| Site | |||||||
| Kenyatta NH | 2,084 | 137(6.6) | 1947(93.4) | 121.6b | <0.001 | - | - |
| Coast PGH | 2,020 | 164(8.1) | 1856(91.9) | ||||
| Nakuru PGH | 1,954 | 214(11.0) | 1740(89.0) | ||||
| Nyeri PGH | 2,890 | 325(11.2) | 2565(88.8) | ||||
| Kakamega PGH | 3,655 | 289(7.9) | 3366(92.1) | ||||
| Embu PGH | 405 | 19(4.7) | 386(95.3) | ||||
| Garissa PGH | 733 | 65(8.9) | 668(91.1) | ||||
| Kakuma | 3,206 | 336(10.5) | 2870(89.5) | ||||
| Dadaab | 2,362 | 284(12.0) | 2078(88.0) | ||||
| Siaya DH | 3,032 | 188(6.2) | 2844(93.8) | ||||
| Nyanza PGH | |||||||
| Length of illness before presenting to the hospital | |||||||
| 0–3 days | 12,996 | 1183(9.1) | 11813(90.9) | Ref | Ref | ||
| 4–7 days | 8,031 | 707(8.8) | 7324(91.2) | 1.0(0.9–1.1) | 0.461 | 1.1(0.9–1.2) | 0.259 |
| >7 days | 1,314 | 131(10.0) | 1183(90.0) | 1.1(0.9–1.3) | 0.300 | 1.3(1.0–1.7) | 0.040 |
| Have children or take care of children in the household (Yes vs. No) | 10,900 | 1024(9.4) | 9876(90.6) | 1.1(1.0–1.2) | 0.122 | 1.1(1.0–1.2) | 0.184 |
| Underlying chronic disease c | |||||||
| Any chronic disease (Yes vs. No) | 1,371 | 125(9.1) | 1246(90.9) | 1.0(0.8–1.2) | 0.644 | - | - |
| Heart disease (Yes vs. No) | 95 | 4(4.2) | 91(95.8) | 0.4(0.2–1.2) | 0.096 | 0.7(0.3–2.1) | 0.571 |
| Chronic shortness of breath (Yes vs. No) | 237 | 17(7.2) | 220(92.8) | 0.7(0.5–1.2) | 0.237 | - | |
| Recurrent chest pain (Yes vs. No) | 458 | 71(15.5) | 387(84.5) | 1.8(1.4–2.3) | <0.001 | 1.3(1.0–1.8) | 0.085 |
| Asthma (Yes vs. No) | 309 | 14(4.5) | 295(95.5) | 0.5(0.3–0.8) | 0.004 | 0.5(0.3–0.9) | 0.023 |
| Chronic cough (Yes vs. No) | 102 | 16(15.7) | 86(84.3) | 1.8(1.1–3.1) | 0.032 | 1.7(0.9–3.1) | 0.084 |
| Active Tb (Yes vs. No) | 53 | 1(1.9) | 52(98.1) | 0.2(0.0–1.4) | 0.096 | 0.3(0.0–2.0) | 0.202 |
| Hospitalized in the last 12 months (Yes vs. No) | 3,576 | 341(9.5) | 3235(90.5) | 1.1(0.9–1.2) | 0.356 | - | |
| Had contact with anyone with a similar illness in the last 3 week (Yes vs. No) | 1,839 | 168(9.1) | 1671(90.9) | 1.0(0.8–1.2) | 0.765 | - | |
| Vaccinated against influenza in the past year (Yes vs. No) | 295 | 22(7.5) | 273(92.5) | 0.9(0.6–1.4) | 0.576 | - | |
| Signs and symptoms within the last 14 days * | |||||||
| Reported or Documented Fever (Yes vs. No) | 18,408 | 1770(9.6) | 16638(90.4) | 1.6(1.4–1.8) | <0.001 | 1.4(1.2–1.8) | <0.001 |
| Weight loss (Yes vs. No) | 5,377 | 399(7.4) | 4978(92.6) | 0.8(0.7–0.8) | <0.001 | 0.8(0.7–0.9) | 0.002 |
| Convulsions (Yes vs. No) | 5,242 | 528(10.1) | 4714(89.9) | 1.2(1.0–1.3) | 0.005 | 1.2(1.0–1.3) | 0.032 |
| Stridor (Yes vs. No) | 4,042 | 400(9.9) | 3642(90.1) | 1.1(1.0–1.3) | 0.027 | 1.0(0.9–1.2) | 0.899 |
| Nasal flaring (Yes vs. No) | 9,482 | 880(9.3) | 8602(90.7) | 1.0(0.9–1.1) | 0.369 | - | |
| Tachypnea (Yes vs. No)c | 6,278 | 561(8.9) | 5717(91.1) | 0.9(0.8–1.0) | 0.194 | 0.9(0.8–1.1) | 0.215 |
| Chest in-drawing | 8,234 | 726(8.8) | 7508(91.2) | 1.0(0.9–1.1) | 0.368 | - | |
| Grunting (Yes vs. No)c | 7,353 | 705(9.6) | 6648(90.4) | 1.1(1.0–1.2) | 0.316 | - | |
| Unable to drink or breastfeed at all | 7,234 | 678(9.4) | 6556(90.6) | 1.1(1.0–1.2) | 0.143 | 1.0(0.9–1.2) | 0.966 |
| Lethargic (Yes vs. No) | 6,370 | 624(9.8) | 5746(90.2) | 1.1(1.0–1.3) | 0.014 | 1.1(0.9–1.3) | 0.263 |
| Loss of consciousness (Yes vs. No) | 1,853 | 218(11.8) | 1635(88.2) | 1.4(1.2–1.6) | <0.001 | 1.0(0.8–1.3) | 0.804 |
| Headache (Yes vs. No) | 769 | 103(13.4) | 666(86.6) | 1.6(1.3–1.9) | <0.001 | 1.2(0.9–1.5) | 0.146 |
| Sore throat (Yes vs. No) | 1,977 | 197(10.0) | 1780(90.0) | 1.1(1.0–1.3) | 0.136 | 1.0(0.8–1.2) | 0.958 |
| Nausea (Yes vs. No) | 4,040 | 419(10.4) | 3621(89.6) | 1.2(1.1–1.3) | 0.002 | 1.0(0.9–1.2) | 0.658 |
| Vomiting (Yes vs. No) | 12,270 | 1131(9.2) | 11139(90.8) | 1.0(1.0–1.1) | 0.324 | - |
Adjusted for variables that were significant at p<.2 in the bivariate analysis and data collection site;
Pearson chi-square;
Data not collected in Siaya DH and Tingwangi;
Row percentages shown.
*Signs such as stridor, nasal flaring, tachypnea, chest in-drawing and grunting were evaluated at the time of patient presentation to the hospital.
Table 3. Odds of testing positive for influenza for various demographic and clinical characteristics among SARI patients aged ≥5 years in Kenya, July 2007–June 2013.
| Total (N = 2,421) | Influenza positive patients (N = 357) | Influenza negative patients (N = 2,064) | Crude OR (95% CI) | p-Value | Adjusteda OR (95% CI) | p-Value | |
| n(%)¥ | n(%)¥ | ||||||
| Sex | |||||||
| Male | 1,295 | 187(14.4) | 1108(85.6) | Ref | |||
| Female | 1,126 | 170(15.1) | 956(84.9) | 1.1(0.8–1.3) | 0.649 | - | |
| Age Group | |||||||
| 5–9 years | 1,247 | 204(16.4) | 1043(83.6) | Ref | Ref | ||
| 10–17 years | 360 | 58(16.1) | 302(83.9) | 1.0(0.7–1.4) | 0.911 | 0.9(0.6–1.3) | 0.484 |
| 18–49 years | 684 | 81(11.8) | 603(88.2) | 0.7(0.5–0.9) | 0.008 | 0.7(0.4–1.1) | 0.086 |
| ≥50 years | 130 | 14(10.8) | 116(89.2) | 0.6(0.3–1.1) | 0.100 | 0.8(0.4–1.8) | 0.627 |
| Site | |||||||
| Kenyatta NH | 54 | 1(1.9) | 53(98.1) | Ref | |||
| Coast PGH | 92 | 7(7.6) | 85(92.4) | 77.8b | <0.001c | - | - |
| Nakuru PGH | 140 | 16(11.4) | 124(88.6) | ||||
| Nyeri PGH | 228 | 34(14.9) | 194(85.1) | ||||
| Kakamega PGH | 645 | 73(11.3) | 572(88.7) | ||||
| Embu PGH | 25 | 0(0.0) | 25(100.0) | ||||
| Garissa PGH | 31 | 9(29.0) | 22(71.0) | ||||
| Kakuma | 499 | 67(13.4) | 432(86.6) | ||||
| Dadaab | 518 | 130(25.1) | 388(74.9) | ||||
| Siaya DH | 93 | 14(15.1) | 79(84.9) | ||||
| Nyanza PGH | 96 | 6(6.3) | 90(93.8) | ||||
| Length of illness before presenting to the hospital | |||||||
| 0–3 days | 1,303 | 215(16.5) | 1088(83.5) | Ref | |||
| 4–7 days | 928 | 125(13.5) | 803(86.5) | 0.8(0.6–1.0) | 0.050 | 1.1(0.8–1.5) | 0.568 |
| >7 days | 190 | 17(8.9) | 173(91.1) | 0.5(0.3–0.8) | 0.008 | 0.7(0.4–1.5) | 0.396 |
| Have children or take care of children in the household (Yes vs. No) | 1,323 | 169(12.8) | 1154(87.2) | 1.2(0.9–1.7) | 0.166 | 1.4(0.9–2.0) | 0.094 |
| Underlying chronic disease d | |||||||
| Any chronic disease (Yes vs. No) | 324 | 31(9.6) | 293(90.4) | 0.6(0.4–0.9) | 0.005 | 0.8(0.5–1.4) | 0.453 |
| Heart disease (Yes vs. No) | 19 | 1(5.3) | 18(94.7) | 0.3(0.0–2.3) | 0.249 | ||
| Chronic shortness of breath (Yes vs. No) | 32 | 3(9.4) | 29(90.6) | 0.6(0.2–1.9) | 0.347 | ||
| Recurrent chest pain (Yes vs. No) | 49 | 9(18.4) | 40(81.6) | 1.2(0.6–2.6) | 0.559 | ||
| Asthma (Yes vs. No) | 61 | 5(8.2) | 56(91.8) | 0.5(0.2–1.2) | 0.127 | 0.8(0.3–2.7) | 0.754 |
| Chronic cough (Yes vs. No) | 42 | 5(11.9) | 37(88.1) | 0.7(0.3–1.9) | 0.528 | ||
| Active Tb (Yes vs. No) | 94 | 6(6.4) | 88(93.6) | 0.4(0.2–0.9) | 0.020 | 1.0(0.3–3.1) | 0.999 |
| Hospitalized in the last 12 months (Yes vs. No) | 450 | 57(12.7) | 393(87.3) | 1.1(0.8–1.5) | 0.612 | ||
| Had contact with anyone with a similar illness in the last 3 week (Yes vs. No) | 46 | 9(19.6) | 37(80.4) | 1.6(0.8–3.3) | 0.227 | ||
| Currently smoking (Yes vs. No) | 112 | 11(9.8) | 101(90.2) | 0.5(0.3–1.0) | 0.056 | 1.1(0.5–2.5) | 0.745 |
| Vaccinated against influenza in the past year (Yes vs. No) | 80 | 7(8.8) | 73(91.3) | 0.7(0.3–1.7) | 0.469 | ||
| Symptoms within the last 14 days | |||||||
| Difficulty in breathing/shortness of breath (Yes vs. No) | 1,850 | 278(15.0) | 1572(85.0) | 1.1(0.8–1.4) | 0.483 | ||
| Sore throat (Yes vs. No) | 805 | 113(14.0) | 692(86.0) | 0.9(0.7–1.2) | 0.488 | ||
| Weight loss (Yes vs. No) | 766 | 91(11.9) | 675(88.1) | 0.7(0.5–0.9) | 0.006 | 0.8(0.5–1.1) | 0.214 |
| Convulsions (Yes vs. No) | 387 | 62(16.0) | 325(84.0) | 1.1(0.8–1.5) | 0.463 | ||
| Lethargic (Yes vs. No) | 493 | 100(20.3) | 393(79.7) | 1.7(1.3–2.1) | <0.001 | 1.1(0.8–1.7) | 0.505 |
| Loss of consciousness (Yes vs. No) | 257 | 58(22.6) | 199(77.4) | 1.8(1.3–2.5) | <0.001 | 1.0(0.6–1.5) | 0.868 |
| Headache (Yes vs. No) | 1,086 | 189(17.4) | 897(82.6) | 1.5(1.2–1.8) | 0.001 | 1.5(1.1–2.0) | 0.018 |
| Nausea (Yes vs. No) | 631 | 113(17.9) | 518(82.1) | 1.4(1.1–1.8) | 0.011 | 1.0(0.7–1.4) | 0.965 |
| Vomiting (Yes vs. No) | 1,187 | 178(15.0) | 1009(85.0) | 1.0(0.8–1.3) | 0.734 | ||
| Muscle pains (Yes vs. No) | 536 | 81(15.1) | 455(84.9) | 1.0(0.8–1.4) | 0.821 | - |
Adjusted for variables that were significant at p<.2 in the bivariate analysis and data collection site;
Pearson chi-square;
Fisher's exact p-value;
Data not collected in Siaya DH and Tingwangi;
Row percentages shown.
Among ILI cases, children ≤23 months old had the lowest percentage of influenza-positive specimens [844/7916 (10.7%] compared to all other age groups. ILI patients who presented to the hospital after 7 days of illness were less likely to be influenza-positive compared to ILI patients with shorter presentations. There were no other demographic characteristics or clinical signs or symptoms that were significantly associated with either influenza-positive or influenza-negative ILI patients (Table 4).
Table 4. Odds of testing positive for influenza for various demographic and clinical characteristics among ILI patients in Kenya, Jul 2007–Jun 2013.
| Total (N = 14,013) | Influenza positive patients (N = 2,041) | Influenza negative patients (N = 11,972) | Crude OR (95% CI) | p-Value | Adjusteda OR (95% CI) | p-Value | |
| n(%)¥ | n(%)¥ | ||||||
| Sex | |||||||
| Male | 7,431 | 1069(14.4) | 6362(85.6) | Ref | |||
| Female | 6,582 | 972(14.8) | 5610(85.2) | 1.0(0.9–1.1) | 0.522 | - | |
| Age Group | |||||||
| 0–23 months | 7,916 | 844(10.7) | 7072(89.3) | Ref | Ref | ||
| 24–59 months | 4,281 | 761(17.8) | 3520(82.2) | 1.8(1.6–2.0) | <0.001 | 1.8(1.6–2.0) | <0.001 |
| 5–9 years | 1,199 | 273(22.8) | 926(77.2) | 2.5(2.1–2.9) | <0.001 | 2.5(2.1–2.9) | <0.001 |
| 10–17 years | 269 | 81(30.1) | 188(69.9) | 3.6(2.8–4.7) | <0.001 | 4.3(3.3–5.8) | <0.001 |
| 18–49 years | 321 | 74(23.1) | 247(76.9) | 2.5(1.9–3.3) | <0.001 | 3.8(2.8–5.2) | <0.001 |
| ≥50 years | 27 | 8(29.6) | 19(70.4) | 3.5(1.5–8.1) | 0.003 | 5.0(2.1–11.9) | <0.001 |
| Site | |||||||
| Kenyatta NH | 1,443 | 168(11.6) | 1275(88.4) | 79.5b | <0.001 | - | - |
| Coast PGH | 809 | 100(12.4) | 709(87.6) | ||||
| Nakuru PGH | 1,869 | 315(16.9) | 1554(83.1) | ||||
| Nyeri PGH | 1,999 | 362(18.1) | 1637(81.9) | ||||
| Kakamega PGH | 2,022 | 325(16.1) | 1697(83.9) | ||||
| Embu PGH | 794 | 68(8.6) | 726(91.4) | ||||
| Garissa PGH | 546 | 91(16.7) | 455(83.3) | ||||
| Kakuma | 1,477 | 191(12.9) | 1286(87.1) | ||||
| Dadaab | 1,303 | 200(15.3) | 1103(84.7) | ||||
| Ting'wang'i HC | 1,649 | 211(12.8) | 1438(87.2) | ||||
| Nyanza PGH | 102 | 10(9.8) | 92(90.2) | ||||
| Length of illness before presenting to the hospital | |||||||
| 0–3 days | 10,588 | 1572(14.8) | 9016(85.2) | Ref | Ref | ||
| 4–7 days | 3,037 | 428(14.1) | 2609(85.9) | 0.9(0.8–1.1) | 0.301 | 1.0(0.8–1.1) | 0.520 |
| >7 days | 388 | 41(10.6) | 347(89.4) | 0.7(0.5–0.9) | 0.020 | 0.7(0.5–0.98) | 0.037 |
| Have children or take care of children in the household | 6,121 | 955(15.6) | 5166(84.4) | 1.2(1.1–1.3) | 0.001 | 1.0(0.9–1.2) | 0.529 |
| Underlying chronic disease c | |||||||
| Any chronic disease (Yes vs. No) | 327 | 51(15.6) | 276(84.4) | 1.1(0.8–1.4) | 0.747 | - | |
| Heart disease (Yes vs. No) | 25 | 4(16.0) | 21(84.0) | 1.1(0.4–3.2) | 0.849 | - | |
| Chronic shortness of breath (Yes vs. No) | 70 | 8(11.4) | 62(88.6) | 0.7(0.4–1.6) | 0.437 | - | |
| Recurrent chest pain (Yes vs. No) | 16 | 4(25.0) | 12(75.0) | 1.9(0.6–6.0) | 0.254 | - | |
| Asthma (Yes vs. No) | 85 | 14(16.5) | 71(83.5) | 1.1(0.6–2.0) | 0.635 | - | |
| Chronic cough (Yes vs. No) | 58 | 10(17.2) | 48(82.8) | 1.2(0.6–2.4) | 0.583 | - | |
| Active Tb (Yes vs. No) | 19 | 4(21.1) | 15(78.9) | 1.6(0.5–4.7) | 0.434 | - | |
| Had contact with anyone with a similar illness in the last 3 week (Yes vs. No) | 1,489 | 236(15.8) | 1253(84.2) | 1.0(0.9–1.2) | 0.578 | - | |
| Hospitalized in last 12 months (Yes vs. No) | 1,356 | 195(14.4) | 1161(85.6) | 0.97(0.8–1.2) | 0.750 | - | |
| Vaccinated against influenza in the past year (Yes vs. No) | 222 | 25(11.3) | 197(88.7) | 0.8(0.5–1.3) | 0.356 | - | |
| Symptoms within the last 14 days | |||||||
| Difficulty in breathing/shortness of breath (Yes vs. No) | 4,094 | 577(14.1) | 3517(85.9) | 0.9(0.9–1.1) | 0.310 | - | |
| Weight loss (Yes vs. No) | 1,732 | 237(13.7) | 1495(86.3) | 0.9(0.8–1.1) | 0.250 | - | |
| Convulsions (Yes vs. No) | 330 | 50(15.2) | 280(84.8) | 1.0(0.8–1.4) | 0.773 | - | |
| Lethargic (Yes vs. No) | 1,108 | 135(12.2) | 973(87.8) | 0.8(0.7–1.0) | 0.019 | 0.8(0.7–1.0) | 0.057 |
| Loss of consciousness(Yes vs. No) | 137 | 16(11.7) | 121(88.3) | 0.8(0.5–1.3) | 0.337 | - | |
| Headache (Yes vs. No) | 1,636 | 327(20.0) | 1309(80.0) | 1.5(1.4–1.8) | <0.001 | 1.1(1.0–1.3) | 0.081 |
| Nausea (Yes vs. No) | 1,795 | 295(16.4) | 1500(83.6) | 1.2(1.0–1.3) | 0.018 | 1.0(0.9–1.2) | 0.621 |
| Vomiting (Yes vs. No) | 2,659 | 369(13.9) | 2290(86.1) | 0.9(0.8–1.1) | 0.264 | - |
Adjusted for variables that were significant at p<.2 in the bivariate analysis and data collection site;
Pearson chi-square;
Data not collected in Siaya DH and Tingwangi;
Row percentages shown.
Of the 9,419 SARI patients who were followed up after being admitted to the hospital, 257 (2.7%) died; 246 (96%) of these deaths occurred within 30 days of admission (Table 5). The median length of stay was 4 days (IQR = 2–6 days) for all SARI patients. The percentage of SARI patients who died varied between hospitals, and SARI patients with underlying chronic disease were more likely to die than those without (Adjusted Relative Risk = 1.9, 95% CI = 1.3–2.8). There was no difference in the duration of hospitalization between influenza-positive and influenza-negative patients. However, fewer influenza-positive patients died compared to influenza-negative patients (0.9% vs. 2.8%, p = 0.010). Of the seven influenza-positive patients who died, six were male, the median age was 1 year (range: 8 months–32 years), and the median number of days between admission and death was 12 days (range 4–19 days). Three had pandemic influenza H1N1, one had H3N2, three had influenza B, and two were influenza A with indeterminate subtyping results.
Table 5. Relative risk of various demographic and clinical characteristics for death within 30 days of admission among SARI patients admitted to 8 hospitals in Kenya, January 2008–June 2013.
| Total (N = 9,339) | Fatal cases (N = 246) | Non-fatal cases (N = 9,093) | Unadjusted RR (95% CI) | p-value | Adjusteda RR (95% CI) | p-value | |
| n(%)¥ | n(%)¥ | ||||||
| Sex | |||||||
| Male | 5,171 | 126(2.4) | 5045(97.6) | Ref | |||
| Female | 4,168 | 120(2.9) | 4048(97.1) | 1.2(0.9–1.5) | 0.185 | 1.1(0.9–1.4) | 0.379 |
| Site | |||||||
| Kenyatta NH | 733 | 57(7.8) | 676(92.2) | Ref | Ref | ||
| Coast PGH | 842 | 17(2.0) | 825(98.0) | 0.3(0.2–0.4) | <0.001 | 0.4(0.2–0.8) | 0.012 |
| Nakuru PGH | 1,065 | 23(2.2) | 1042(97.8) | 0.3(0.2–0.4) | <0.001 | 0.5(0.3–0.9) | 0.013 |
| Nyeri PGH | 1,648 | 25(1.5) | 1623(98.5) | 0.2(0.1–0.3) | <0.001 | 0.3(0.2–0.5) | 0.000 |
| Kakamega PGH | 1,783 | 17(1.0) | 1766(99.0) | 0.1(0.1–0.2) | <0.001 | 0.2(0.1–0.5) | 0.000 |
| Embu PGH | 153 | 5(3.3) | 148(96.7) | 0.4(0.2–1.0) | 0.058 | 0.8(0.3–2.3) | 0.726 |
| Garissa PGH | 68 | 6(8.8) | 62(91.2) | 1.1(0.5–2.5) | 0.758 | 1.5(0.8–3.1) | 0.207 |
| Siaya DH | 3,047 | 96(3.2) | 2951(96.8) | 0.4(0.3–0.6) | <0.001 | 0.4(0.1–1.7) | 0.225 |
| Length of illness before presenting to hospital | |||||||
| 0–3 days | 4,745 | 97(2.0) | 4648(98.0) | Ref | Ref | ||
| 4–7 days | 3,901 | 114(2.9) | 3787(97.1) | 1.4(1.1–1.9) | 0.009 | 1.3(1.0–1.7) | 0.066 |
| >7 days | 693 | 35(5.1) | 658(94.9) | 2.5(1.7–3.6) | <0.001 | 1.6(1.1–2.3) | 0.023 |
| Duration of hospitalization | |||||||
| 0–3 days | 4,466 | 113(2.5) | 4353(97.5) | Ref | Ref | ||
| 4–7 days | 3,724 | 82(2.2) | 3642(97.8) | 0.9(0.7–1.2) | 0.332 | 0.7(0.6–1.0) | 0.028 |
| >7 days | 1,149 | 51(4.4) | 1098(95.6) | 1.8(1.3–2.4) | 0.001 | 0.9(0.6–1.2) | 0.442 |
| Influenza test | |||||||
| Tested negative | 8,585 | 239(2.8) | 8346(97.2) | Ref | |||
| Tested positive | 754 | 7(0.9) | 747(99.1) | 0.3(0.2–0.7) | 0.004 | 0.4(0.2–0.8) | 0.010 |
| Underlying chronic disease | |||||||
| No underlying chronic disease | 5732 | 114(2.0) | 5618(98.0) | Ref | |||
| Reported any underlying chronic disease | 525 | 34(6.5) | 491(93.5) | 3.3(2.2–4.7) | <0.001 | 1.9(1.3–2.8) | 0.001 |
| Signs and symptoms on admission * | |||||||
| Reported or documented fever (Yes vs. No) | 7,954 | 209(2.6) | 7745(97.4) | 1.0(0.7–1.4) | 0.925 | - | |
| Difficulty in breathing/shortness of breath (Yes vs. No) | 6,637 | 172(2.6) | 6465(97.4) | 0.9(0.7–1.2) | 0.687 | - | |
| Weight loss (Yes vs. No) | 3,004 | 145(4.8) | 2859(95.2) | 3.2(2.5–4.1) | <0.001 | 2.1(1.6–2.9) | 0.000 |
| Convulsions (Yes vs. No) | 2,413 | 49(2.0) | 2364(98.0) | 0.7(0.5–1.0) | 0.032 | 0.8(0.6–1.1) | 0.169 |
| Stridor (Yes vs. No)b | 1,433 | 49(3.4) | 1384(96.6) | 1.4(1.0–2.0) | 0.037 | 1.0(0.7–1.4) | 0.891 |
| Nasal flaring (Yes vs. No)b | 4,175 | 110(2.6) | 4065(97.4) | 1.1(0.8–1.5) | 0.447 | - | |
| Tachypnea (Yes vs. No)b | 2,098 | 69(3.3) | 2029(96.7) | 2.0(1.4–2.8) | <0.001 | 1.0(0.6–1.5) | 0.841 |
| Chest in-drawing (Yes vs. No)b | 3,552 | 98(2.8) | 3454(97.2) | 1.2(0.9–1.6) | 0.159 | 1.5(1.1–2.0) | 0.016 |
| Grunting (Yes vs. No)b | 2,476 | 80(3.2) | 2396(96.8) | 2.5(1.7–3.7) | <0.001 | 1.6(1.0–2.7) | 0.070 |
| Unable to drink or breastfeed at all (Yes vs. No)b | 2,391 | 61(2.6) | 2330(97.4) | 1.1(0.8–1.4) | 0.711 | - | |
| Lethargic (Yes vs. No) | 2,694 | 105(3.9) | 2589(96.1) | 1.8(1.4–2.4) | <0.001 | 1.2(0.9–1.6) | 0.265 |
| Loss of consciousness (Yes vs. No) | 664 | 32(4.8) | 632(95.2) | 2.0(1.4–2.8) | <0.001 | 2.2(1.4–3.3) | 0.000 |
| Headache (Yes vs. No) | 436 | 22(5.0) | 414(95.0) | 2.0(1.3–3.1) | 0.001 | 1.9(1.2–3.0) | 0.011 |
| Nausea (Yes vs. No) | 1,058 | 44(4.2) | 1014(95.8) | 1.7(1.2–2.4) | 0.001 | 1.2(0.8–1.8) | 0.273 |
| Vomiting (Yes vs. No) | 4,681 | 105(2.2) | 4576(97.8) | 0.7(0.6–1.0) | 0.018 | 0.9(0.6–1.1) | 0.311 |
Adjusted for variables that were significant at p<.2 in the bivariate analysis and data collection site;
Applies only for children <5 years;
Row percentages shown.
*Signs such as stridor, nasal flaring, tachypnea, chest in-drawing and grunting were evaluated at the time of patient presentation to the hospital.
Discussion
This is the first report of national influenza sentinel surveillance in Kenya. We found that throughout six years of surveillance, influenza was an important contributor to both inpatient and outpatient acute respiratory illness. Indeed, influenza was associated with nearly one-tenth of all hospitalized SARI cases, and almost one-sixth of all outpatient ILI cases. Over half of patients enrolled in our national SARI and ILI surveillance system were children less than two-years old. Although the percent-positivity for influenza was higher for both SARI and ILI in older age groups, the absolute number of influenza cases was greatest in children under age two. Similar findings were recently reported in a description of influenza surveillance in 15 African countries [10], including Kenya, from 2006–2010. Other reports from Kenya [2], [15]–[17] and studies from other resource-poor countries, like Bangladesh and Lao PDR [18], [19], have also shown that young children bear a heavy burden of influenza-associated respiratory disease.
In Kenya, influenza vaccine is rarely used [15], and in our surveillance system, although slightly more than one percent of patients had been vaccinated against influenza in the prior year, most of these patients were from Kakuma Refugee Camp and were vaccinated in 2010 as part of a free seasonal influenza vaccination campaign that was offered in the camp. Young children <2 years old could be a logical initial target population if a nationwide influenza immunization program is implemented in the future. Maternal immunization could also be considered to protect infants <6 months old [20]. Additionally, we found that nearly one-sixth of all influenza-associated SARI patients had been hospitalized in the previous 12 months, a trend that has also been documented in studies in the United States [21]. We observed this same trend among influenza-negative SARI patients. Hospitalized patients, although a much smaller population, could also be a cost-effective target for vaccination in Kenya if more influenza vaccine were to become available in the country.
Although we conducted surveillance over six years, a relatively robust time period to detect seasonal trends, we were not able to detect a consistent, discrete seasonality to influenza activity in Kenya. However, in most years, influenza activity was most pronounced during July to November, a period of low precipitation, relatively lower temperatures, and variable humidity in Kenya [22], [23], and activity was minimal during December and January, which tend to be the warmest months of the year in Kenya [22]. This same seasonal influenza pattern has been described in neighboring Uganda [24]. However, in Kenya, influenza circulated year-round and was detected during a minimum of ten months per year throughout the six year period. In some years, like 2011, influenza also circulated early in the year, during a period typical of high temperatures and heavy rains. Year-round influenza activity has been reported in other tropical countries [25]. Although the trend of increased influenza activity in the middle of the calendar year would support the use of southern hemisphere vaccine in Kenya, the relatively continuous circulation of influenza combined with the variable seasonal peaks in some years complicates this recommendation. In addition, information about the concordance between circulating strains in Kenya and southern and northern hemisphere vaccine strains, which is being evaluated in a separate analysis, will be essential to this recommendation.
In Kenya, influenza types and subtypes co-circulated through the six years of surveillance. Following the introduction of influenza A (H1N1)pdm 09 in 2009, through 2013, H3N2, A (H1N1) pdm 09, and influenza B co-circulated in Kenya, a trend that was also seen in neighboring Uganda and Rwanda [26].
We found that influenza was associated with deaths and was responsible for a considerable burden on hospitals. Of the nearly 10,000 hospitalized SARI patients we followed, 0.9% of the 754 influenza-positive SARI patients died. This case-fatality ratio for influenza-associated hospitalizations is substantial. It is higher than estimates of in-hospital case-mortality rates for children in the United States [27] and Thailand [3], although it is lower than rates recently reported for seasonal and pandemic influenza in India [28]. This may reflect a difference in underlying chronic illness or acute conditions, such as HIV or malaria, which we did not routinely test for in SARI or ILI patients. In our surveillance population, the median age was very young (16 months), and the median age of patients who died was 2 years old; this contrasts with the findings from the studies in Thailand and India, where most patients who died were adults, and many were older than 50. While there were not any reports of patients who refused to be consented, it is possible that sicker patients, particularly those who died in the hospital, were not enrolled. If sick patients came into the hospital in the evening or on weekends and died in the hospital before the next weekday morning, we would not have captured them in our surveillance system. Additionally, we would have missed patients who died shortly after arriving in the hospital, before even being admitted to a hospital ward. Therefore the CFRs we report for SARI in general and influenza-associated SARI may be under-estimates. The estimated CFR should not have been affected by the surveillance project at the hospital; surveillance officers evaluated patients on admission but were not involved in patient management.
Less than 3% of SARI patients we captured were adults; while this may reflect a lower burden of severe influenza in adults in Kenya, it likely reflects a component of relatively lower health clinic utilization in this age group [29], [30]. This disparity may also reflect the requirement of measured fever in the case definition of SARI in patients >5 years old; a recently published longitudinal cohort study in England showed that only a minority of people with PCR-confirmed influenza had fever with a temperature greater than 37.8°C[31]. Finally, the difference may reflect a possible sampling bias in our surveillance system; surveillance officers may have preferentially captured children in pediatric wards, although they were instructed to routinely survey all wards. In our surveillance system in Kenya, the median duration of hospitalization was 4 days for influenza-positive patients. This length of stay is similar to results from studies in the United States, India and Thailand [3], [27], [28].
Our findings are subject to several limitations. First, the case definition for SARI was different for children <5 years old and persons ≥5 years old, which limited our ability to compare SARI patients across age groups. In addition, we know from previous studies in Kenya that the SARI case definition does not capture all influenza cases [17], [32]; therefore we likely missed some hospitalized influenza cases. Second, we only sampled the first three ILI patients of the day. This could have biased our ILI findings towards sicker patients or patients who lived closer to the health facilities and arrived at the clinic earlier. We did not collect data regarding non-enrolled ILI patients, which would have helped us to better understand how representative sampled ILI patients were of all ILI patients. Furthermore, our surveillance system did not include specialty wards like obstetrics; therefore we were not able to collect data on the relative burden of influenza among pregnant women, a priority group for vaccination [33]. Given that pregnant women can transfer protective antibodies to infants, for whom no influenza vaccine is currently available [20], future surveillance in Kenya should monitor pregnant women. In addition, for the last 18 months, because of budgetary constraints, we scaled back our surveillance to include only nine hospitals, all but two of which conducted SARI surveillance only. Therefore, results for this period were likely not as nationally representative compared to the previous results. This change to a more limited surveillance system reflects the sustainability challenges of conducting surveillance, particularly in resource-poor settings. Finally, our surveillance system was facility-based rather than population-based. Because we were not able to determine catchment areas for each site, and because health utilization practices vary considerably in different parts of Kenya [29], [30], we were not able to determine the overall incidence of influenza in the population. We were also not able to determine the relative hospital burden of influenza because we did not collect data on overall admissions.
We found that during six years influenza contributed to hospitalized respiratory illness and outpatient ILI in all age groups year-round in 11 sites across Kenya. The absolute burden of influenza-associated respiratory illness was highest in children <2 years old; this group could be considered for prioritization for vaccine. While influenza circulated year-round in Kenya, during most years, influenza activity peaked during the peak of the southern hemisphere influenza season. Additional years of surveillance will be helpful in order to better understand the seasonality of influenza in Kenya and inform potential vaccine recommendations.
Acknowledgments
Disclaimer: The findings and conclusions in this paper are those of the author(s) and do not necessarily represent the views of the Centers for Disease Control and Prevention.
We would like to thank Rachel Eidex and Nina Marano for their support and input regarding surveillance, and the International Rescue Committee-Kenya for coordinating surveillance at the refugee sites. We appreciate the efforts of the KEMRI/CDC-Kenya data staff, including Geoffrey Arunga, Dennis Kinyanjui, Christine Ngwawe, and the KEMRI/CDC-Kenya laboratory staff, including Lilian Mayieka, Gilbert Kikwai, Lilian Wakhule and Eunice Mailu, and the surveillance officers, including Beverly Were, Conrad Maseno, Milka Bunei, Molly Mugabe, Peter Kinuthia, Ronald Ondieki, Benjamin Matei, Jonathan Orinda, Esther Nyachomba, Margaret Nguhi, Abdirahman Mahamud, Adan Tepo, Faarax Xassan, Farax Cabdi and Xassan Yare. We thank Kathleen Morales, Carolyn Baer, Evelyne Mulama and Joy Kuboka for their strong logistical support of this project, and Emmaculate Lebo and Peninah Munyua for their periodic technical support of the system. We are grateful to Jerome Tokars for his thorough review of the manuscript.
Funding Statement
The surveillance described in this manuscript was funded by a cooperative agreement between the US Centers for Disease Control and the Kenya Ministry of Health. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Nair H, Brooks WA, Katz M, Roca A, Berkley JA, et al. (2011) Global burden of respiratory infections due to seasonal influenza in young children: a systematic review and meta-analysis. Lancet 378: 1917–1930. [DOI] [PubMed] [Google Scholar]
- 2. Onyango CO, Njeru R, Kazungu S, Achilla R, Bulimo W, et al. (2012) Influenza surveillance among children with pneumonia admitted to a district hospital in coastal Kenya, 2007–2010. J Infect Dis 206 Suppl 1: S61–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Simmerman JM, Chittaganpitch M, Levy J, Chantra S, Maloney S, et al. (2009) Incidence, seasonality and mortality associated with influenza pneumonia in Thailand: 2005–2008. PLoS One 4: e7776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Freitas FT, Souza LR, Azziz-Baumgartner E, Cheng PY, Zhou H, et al. (2012) Influenza-associated excess mortality in southern Brazil, 1980–2008. Epidemiol Infect 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Breiman RF, Nasidi A, Katz MA, Kariuki Njenga M, Vertefeuille J (2007) Preparedness for highly pathogenic avian influenza pandemic in Africa. Emerg Infect Dis 13: 1453–1458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Schoub BD, McAnerney JM, Besselaar TG (2002) Regional perspectives on influenza surveillance in Africa. Vaccine 20 Suppl 2: S45–46. [DOI] [PubMed] [Google Scholar]
- 7. Schoub BD (2010) Surveillance and management of influenza on the African continent. Expert Rev Respir Med 4: 167–169. [DOI] [PubMed] [Google Scholar]
- 8. Gessner BD, Shindo N, Briand S (2011) Seasonal influenza epidemiology in sub-Saharan Africa: a systematic review. Lancet Infect Dis 11: 223–235. [DOI] [PubMed] [Google Scholar]
- 9. Ortu G, Mounier-Jack S, Coker R (2008) Pandemic influenza preparedness in Africa is a profound challenge for an already distressed region: analysis of national preparedness plans. Health Policy Plan 23: 161–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Radin JM, Katz MA, Tempia S, Talla Nzussouo N, Davis R, et al. (2012) Influenza surveillance in 15 countries in Africa, 2006–2010. J Infect Dis 206 Suppl 1: S14–21. [DOI] [PubMed] [Google Scholar]
- 11. Thompson MG, Breiman RF, Hamel MJ, Desai M, Emukule G, et al. (2012) Influenza and malaria coinfection among young children in western Kenya, 2009–2011. J Infect Dis 206: 1674–1684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Organization WH (2011) Integrated Management of Childhood Illness.
- 13.Organization WH (2006) World Health Organization (2006) Collecting, preserving and shipping specimens for the diagnosis of avian influenza A(H5N1) virus infection. Guide for field operations. Available: .
- 14. Kim C, Ahmed JA, Eidex RB, Nyoka R, Waiboci LW, et al. (2011) Comparison of nasopharyngeal and oropharyngeal swabs for the diagnosis of eight respiratory viruses by real-time reverse transcription-PCR assays. PLoS One 6: e21610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Katz MA, Lebo E, Emukule G, Njuguna HN, Aura B, et al. (2012) Epidemiology, seasonality, and burden of influenza and influenza-like illness in urban and rural Kenya, 2007–2010. J Infect Dis 206 Suppl 1: S53–60. [DOI] [PubMed] [Google Scholar]
- 16. Berkley JA, Munywoki P, Ngama M, Kazungu S, Abwao J, et al. (2010) Viral etiology of severe pneumonia among Kenyan infants and children. JAMA 303: 2051–2057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Feikin DR, Ope MO, Aura B, Fuller JA, Gikunju S, et al. (2012) The population-based burden of influenza-associated hospitalization in rural western Kenya, 2007–2009. Bull World Health Organ 90: 256–263A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Azziz-Baumgartner E, Alamgir AS, Rahman M, Homaira N, Sohel BM, et al. (2012) Incidence of influenza-like illness and severe acute respiratory infection during three influenza seasons in Bangladesh, 2008–2010. Bull World Health Organ 90: 12–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Sentilhes AC, Choumlivong K, Celhay O, Sisouk T, Phonekeo D, et al. (2013) Respiratory virus infections in hospitalized children and adults in Lao PDR. Influenza Other Respi Viruses [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Zaman K, Roy E, Arifeen SE, Rahman M, Raqib R, et al. (2008) Effectiveness of maternal influenza immunization in mothers and infants. N Engl J Med 359: 1555–1564. [DOI] [PubMed] [Google Scholar]
- 21. Zerr DM, Englund JA, Robertson AS, Marcuse EK, Garrison MM, et al. (2008) Hospital-based influenza vaccination of children: an opportunity to prevent subsequent hospitalization. Pediatrics 121: 345–348. [DOI] [PubMed] [Google Scholar]
- 22.(2013) World Weather Online. pp.Monthly average weather and rainfall in Kenya.
- 23.(2013) BBC Weather Nairobi.
- 24. Lutwama JJ, Bakamutumaho B, Kayiwa JT, Chiiza R, Namagambo B, et al. (2012) Clinic- and hospital-based sentinel influenza surveillance, Uganda 2007–2010. J Infect Dis 206 Suppl 1: S87–93. [DOI] [PubMed] [Google Scholar]
- 25. Viboud C, Alonso WJ, Simonsen L (2006) Influenza in tropical regions. PLoS Med 3: e89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Organization WH (2013) FluNet.
- 27. Thompson WW, Shay DK, Weintraub E, Brammer L, Bridges CB, et al. (2004) Influenza-associated hospitalizations in the United States. JAMA 292: 1333–1340. [DOI] [PubMed] [Google Scholar]
- 28. Chudasama R, Patel U, Verma P, Banerjee A, Buch P, et al. (2013) A Two Wave Analysis of Hospitalizations and Mortality from Seasonal and Pandemic 2009 A (H1N1) Influenza in Saurashtra, India: 2009–2011. Ann Med Health Sci Res 3: 334–340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Breiman RF, Olack B, Shultz A, Roder S, Kimani K, et al. (2011) Healthcare-use for major infectious disease syndromes in an informal settlement in Nairobi, Kenya. J Health Popul Nutr 29: 123–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Burton DC, Flannery B, Onyango B, Larson C, Alaii J, et al. (2011) Healthcare-seeking behaviour for common infectious disease-related illnesses in rural Kenya: a community-based house-to-house survey. J Health Popul Nutr 29: 61–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Hayward AC, Fragaszy EB, Bermingham A, Wang L, Copas A, et al. (2014) Comparative community burden and severity of seasonal and pandemic influenza: results of the Flu Watch cohort study. Lancet Respir Med [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Murray EL, Khagayi S, Ope M, Bigogo G, Ochola R, et al. (2013) What are the most sensitive and specific sign and symptom combinations for influenza in patients hospitalized with acute respiratory illness? Results from western Kenya, January 2007–July 2010. Epidemiol Infect 141: 212–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Vaccines against influenza WHO position paper - November 2012. Wkly Epidemiol Rec 87: 461–476. [PubMed] [Google Scholar]